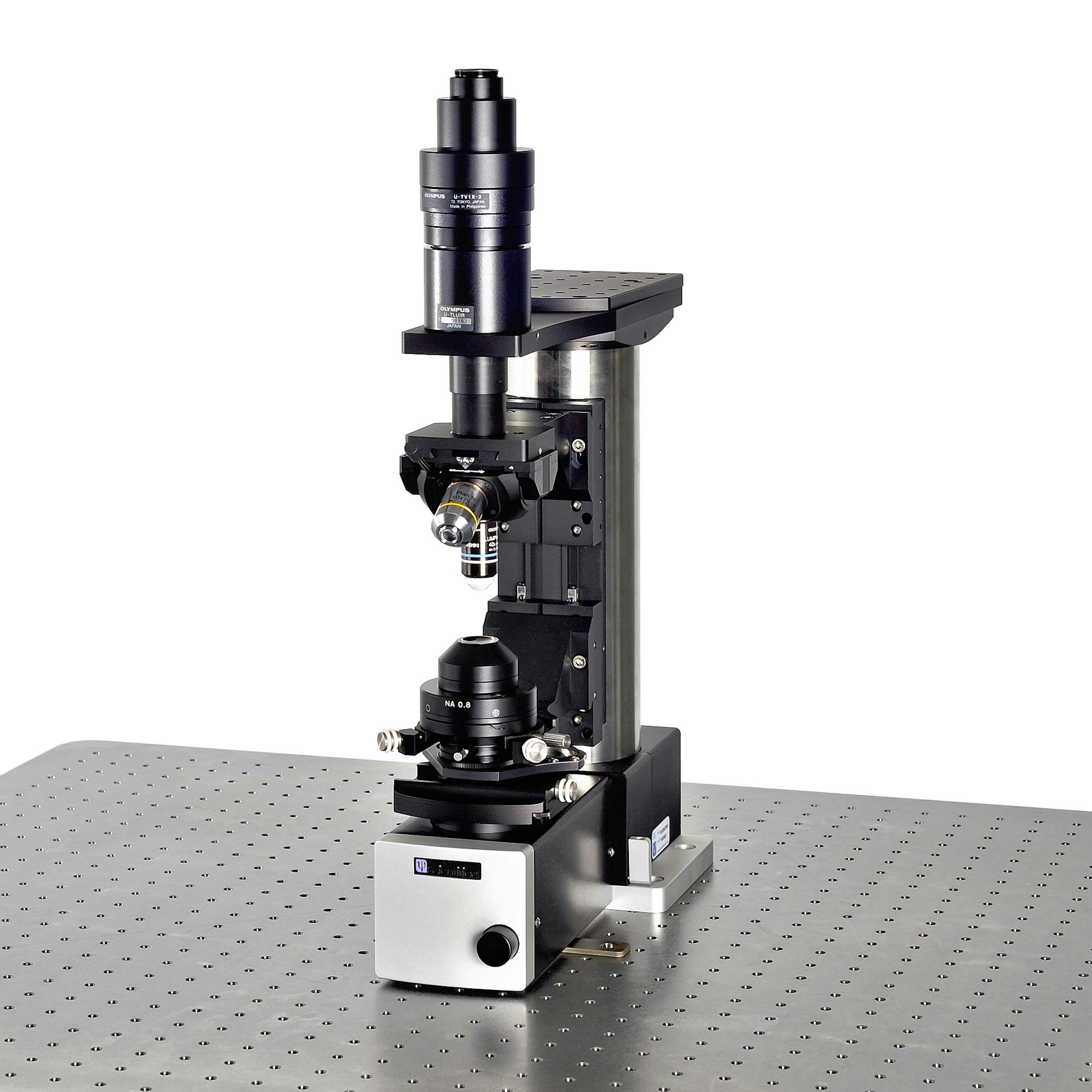
Scientifica SliceScope
An advanced slim microscope designed to support diverse and demanding electrophysiology and imaging experiments.
The Scientifica SliceScope is an ultra-stable, versatile upright microscope. The unbeatable small footprint, low noise electronics, simple conversion between in vitro and in vivo as well as the remote control, have made the SliceScope an integral part of electrophysiology rigs around the world.
Product benefits

Control options
Control the focus, condenser, translation stage (if included) from any of our remote control options.
Alternatively use Scientifica’s LinLab software, developed specifically to control all of our motorised components and heating and perfusion elements.
Testimonials
Confidently control your electrophysiology experiments with the fully integrated SliceScope Pro systems.
Perfect foundation for many electrophysiology rigs
The SliceScope forms part of the Scientifica SliceScope Pro Systems which are fully integrated electrophysiology rigs. These versatile systems are available as four main packages which all include the SliceScope, PatchStar manipulators and Scientifica staging, but depending on your experimental needs and personal preferences, the SliceScope Pro systems can be fully tailored to suit you.
How customers are using the SliceScope
Scientifica services for this product
Speak to one of our experts for details on pricing, features, installation and support.