
#LabHacks: Tips for performing adult animal brain slicing for patch clampers
By Jonathan Ting, PhD
Preparing consistently healthy acute brain slices from mature adult animals for patch clamping experiments can be challenging. Nonetheless, the use of mature animals is often scientifically justified, such as for experiments aimed at charting the emergence of intrinsic or synaptic dysfunction as a function of animal age (e.g. comparison of wild-type and gene knockout mice), or for characterising cell properties without confounds of incomplete development and maturation. Design your experiments with the animal cohort and age that is most appropriate to address your key scientific questions, not based on the animal age that is easiest for brain slicing. By following these simple tips, your adult brain slices can be better than ever, and you will be ready to tackle the most challenging of patch clamp experiments.
1. Invest in a quality slicer machine
The goal is to best preserve the neurons in the slice (both superficial and deep) for patch clamp recording. Various tissue slicer models are commercially available, many of which can provide excellent performance when optimally calibrated. The ideal instrument should have minimal z-axis deflection, either directly measured and tuned or empirically observed. Adult brain tissue is firmer than juvenile brain tissue with highly developed myelination. In some cases, use of a special blade type in conjunction with the high-quality slicer machine may be advantageous to provide higher uniformity of sectioning and to avoid tissue compression during slicing. Some specialty blades, like Zirconium ceramic blades, can also be reused numerous times without loss in performance or dulling, even for adult brain tissue
2. Adopt media formulations optimised on adult brain tissue
Adult brain tissue is more susceptible to damage from a variety of different insults during the slicing procedure. Whereas a juvenile animal brain might tolerate the occasional procedural mistake, the adult brain tissue is generally far less forgiving. To overcome this disparity, a more highly neuroprotective aCSF formulation can help a ton. Not all slicing solutions are created equal. Try an NMDG aCSF formulation where NMDG-Cl is substituted in place of NaCl. The addition of 20 mM HEPES is also an effective way to provide stronger pH buffering in the physiological range and to prevent excessive oedema. A 0.5 mM Ca/10 mM Mg ratio will ensure that neural excitability is very low. Finally, supplements for energy supply (pyruvate) and antioxidant function (ascorbate and thiourea) are added to maintain viability and counter oxidative stress. A full list of recommended aCSF formulations can be found here1.
3. Keep it fresh
Everyone wants to be efficient and save time each day. However, this is one area where shortcuts could be counter-productive. To ensure the best possible results for important experiments, it is preferable to make all aCSF solutions daily and use within 24 hr. In addition, it is critical to use purified water that is free of contaminants such as trace metals. Such impurities that are often found in distilled water sources are damaging to the slices and can convert anti-oxidants such as ascorbate into pro-oxidants.
4. Slow down brain metabolism ASAP
Transcardial perfusion is a reliable way of clearing the blood out of the brain and, in doing so, rapidly slowing metabolic activity by introducing low Na+, low Ca2+ and high Mg2+ NMDG aCSF through the vasculature. This is a more proactive method than removing the whole brain and simply submerging it in pre-chilled cutting solution. For the most consistent results, the transcardial perfusion step is highly recommended.
To the extreme limits: patch clamp recordings in brain slices from 2.5 year old VGAT-ChR2 mice.
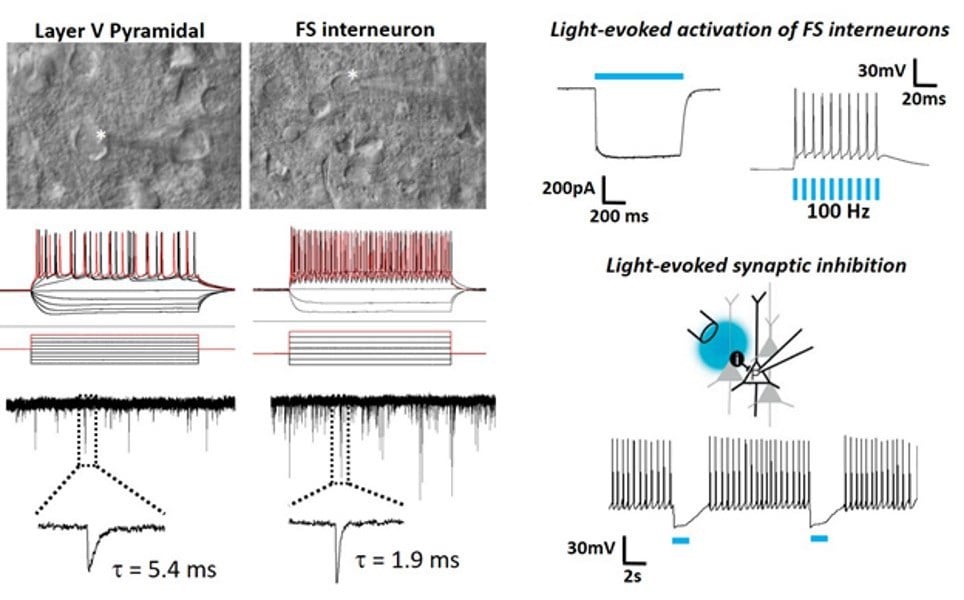
Patch clamp recordings from a pyramidal neuron and fast spiking interneuron in neocortical slices derived from a 2.5 year old VGAT-ChR2-EYFP transgenic mouse with examples of optically evoked firing and synaptic inhibition.
5. Test drive the “Protective Recovery” method
The exact procedure and timing matters more than the choice of slicing solution. Perhaps the most impactful single step is to implement a “protective recovery” period immediately following physical slicing2. By allowing slices to recover from the trauma of slicing for ≤12 minutes in NMDG aCSF before transferring slices into high Na+ containing aCSF, neurons will sustain far less damage and survive in higher proportions. The exact duration of the recovery period is critical for obtaining the optimal balance between morphological and functional preservation of the brain slices. Note that excessively long recovery in NMDG aCSF will result in excellent morphological preservation but poor functional recovery. Shorter recovery in NMDG aCSF will result in more complete functional recovery but poorer morphological preservation.
6. Hold it steady
Don’t underestimate the importance of the slice holding chamber design. After all, most slices will spend more time waiting in this chamber than in your rig recording chamber. A high-quality holding chamber design with a large reservoir size is optimal, for example, the commercial Brain Slice Keeper design. The presence of 20 mM HEPES and ascorbate/thiourea in the aCSF used in the holding chamber reduces slice oedema and slows deterioration over prolonged storage times.
7. Seeing is believing
When it comes to visualising all those healthy neurons in your brain slices, you will want to have your rig equipped with a 900 nm IR bandpass filter (as opposed to a lower wavelength IR bandpass filter) and optimised for Koehler illumination to get the full effect. The ability to see clearly including very deep into the tissue will be of great benefit for monitoring the patch electrode contact with the target cell and visualising the dimple on the membrane surface immediately prior to releasing the positive pressure. The appearance of the cell membrane under IR-DIC optics is often the best predictor of cell health, so better optics also means better discrimination of the healthiest neurons for patching and with higher confidence.
8. Internal solution impacts gigaseal formation
Empirical testing in adult brain slices revealed that gigaohm seals form more quickly with Gluconate based internal solutions (e.g. K-Gluconate or Cs-Gluconate) relative to Methane sulfonate based internal solution, after accounting for pH and osmolarity. The basis for this observed difference is not known, but as such Gluconate-based internal solution is preferred.
References
- brainslicemethods.com
- Ting J.T., Daigle T.L., Chen Q., Feng G. (2014) Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics Methods in Molecular Biology doi: 10.1007/978-1-4939-1096-0_14
About the author
Dr Jonathan Ting is a researcher at the Allen Institute for Brain Science. He is currently leading the effort to develop experimental platforms for functional studies on adult human ex vivo brain slices derived from surgically-excised brain tissue. He is also one of many team members involved in the development of the Allen Cell Types Database, an open-access web resource of patch clamp recording experiments from adult mouse brain slices using highly standardised methodologies to characterise the diversity of morpho-electric cell types in the neocortex. The Allen Institute electrophysiology teams are utilising Scientifica equipment (including PatchStar and MicroStar micromanipulators, SliceScopes, and various other custom hardware solutions) to conduct these high-throughput experiments.
Read other #LabHacks articles
- #LabHacks: How to align your laser for two-photon imaging
- #LabHacks: Tips for cleaning the optics of your microscope
- #LabHacks: To compensate or not to compensate, that is the question
- #LabHacks: How to reduce the noise around your electrophysiology rig
- #LabHacks: Choosing the best opsin for your optogenetics experiments
- #LabHacks: 14 sharp tips for patch clamping

)