
SciMethods: Visual Stimulation of Retinal Explants on a Standard Multiphoton Microscope
Christian Wilms1, Jason Jacoby2, Greg Schwartz2
1 Scientifica Ltd. Uckfield, UK
2 Northwestern University, Chicago, USA
Introduction
The retina is one of the most studied and arguably best understood neuronal circuits in vertebrates. The fact that the retina presents a self-contained sensory circuit (not receiving any neuronal inputs from other sensory or brain regions) makes it an ideal preparation not just for interrogating visual processing in the retina itself, but also for understanding general principles of signal processing and neural computations performed throughout the nervous system.
One of the key advantages of the retina as a model circuit is the ease with which physiological stimuli can be applied to tissue explants. Such explants contain the primary sensory cells (photoreceptors), fully intact downstream circuitry, and at the final stage, output cells of the retina (retinal ganglion cells; RGCs) whose axon projections leave the retina and transmit processed information to higher brain regions. Thus, it is possible to project visual stimuli directly onto the explant and obtain fully physiological responses to this stimulation, including the processing steps within the retinal circuitry and patterns of retinal output in the form of action potentials. One of the advantages of visual stimuli is that the stimulation parameters (pattern, timing, wavelength, location) can be controlled very precisely by the experimenter. In addition, the nature of the actual stimulus can also be defined for assessing the processing of different stimulus properties (e.g. motion, size, contrast, spatial frequency).
To fully understand the function of the retina, it is necessary to assess the role of all cell types in the circuitry. With over 100 different neuron types, the retina has a large neuronal diversity1–3. Many of these different cell types are rare, representing far less than 1% of the total population. Thus, simply recording from cells identified by their location and morphology using light microscopy would not allow a full description of the circuit. With the growing availability of transgenic mouse lines, in which genetically defined subpopulations of neurons are fluorescently labelled, it has become possible to target even very rare cell types reliably.
Using standard (one-photon excited) fluorescence microscopy to visualise fluorescently labelled cells is not a viable option in retinal research, as the intense visible excitation light would cause substantial excitation of the photoreceptors, activating the entire circuitry of the retina, potentially seriously disrupting the experiment or rendering the tissue unresponsive to light. In contrast, two-photon microscopy4 relies on light in the near infrared spectral region (commonly over 800 nm). In two-photon microscopy, the activation of photoreceptors by light excitation is mainly limited to the focus of the microscope, leading to substantially lower levels of activation5. Thus, two-photon targeted recordings6 have become a central method in retinal research7.
In this technical note, we describe two approaches to integrating visual stimulation of the retina into a two-photon microscope.
Implementation
In the Scientifica SliceScope, as in most modern microscopes, visual stimulation can be projected onto the retina either through the condenser (from below) or through the objective (from above). The ‘through the condenser’ approach is the most commonly used, but both methods have their merits and are easy to implement. ‘Through the condenser’ can be easily configured to allow functional imaging and visual stimulation to be performed simultaneously. ‘Through the objective’ allows visual stimuli to pass through the inner layer of the retina, as during natural activation of the retina in the intact eye. In addition, the experimenter can choose to leave the retinal pigment epithelium and sclera undamaged, protecting the photoreceptors from excessive photobleaching. Implementing both of these approaches on the same microscope increases the number of wavelengths that can be used at the same time, further extending the experimental capabilities.
Stimulus generation
Visual stimulation can be considered as two steps: the stimulation pattern needs to be generated, and it needs to be projected.
Stimulus patterns are almost exclusively generated using a personal computer running a dedicated software package. In principle, any software package capable of generating patterns and movies can be used. But commonly, integrated programming environments are the tool of choice, due to their powerful means of generating well-defined patterns in pixel-space and time. Frequently used packages include the Psychophysics Toolbox (http://psychtoolbox.org/) and Stage-VSS (http://stage-vss.github.io/), both running in the Matlab environment (Mathworks).
Projection of the stimulus pattern is achieved with a compact programmable projector such as the LightCrafter (Texas Instruments). In most compact light projectors, light from LEDs is projected onto a digital micromirror device (DMD). This DMD is the component of the projector that generates the actual pixel pattern. Simplified, a DMD is an array of very small mirrors that can be toggled between two positions. Tilted to the ‘on’ position, they reflect light to the projector output and the corresponding pixel appears bright; tilted to an ‘off’ position, the light is reflected to a beam dump and the corresponding pixel appears dark (Texas Instruments provide a video with more information (see above). In compact projectors, multicolour projections are achieved by sequentially illuminating a single DMD with different colour LEDs. Most compact light projectors come preconfigured with a three LED light source (red, green, blue). In most cases, it is possible to either change the LED colour selection yourself or purchase a customised version through a thirdparty supplier (e.g. EKB Technologies). Ideally, the selected LEDs will match the spectral properties of the photoreceptors of the species being studied or the circuit being targeted. In the case of the murine retina, exchanging the red LED for a near UV-emitting LED has proven advantageous8,9. A schematic of a DMD based projector is shown in Figure 1.
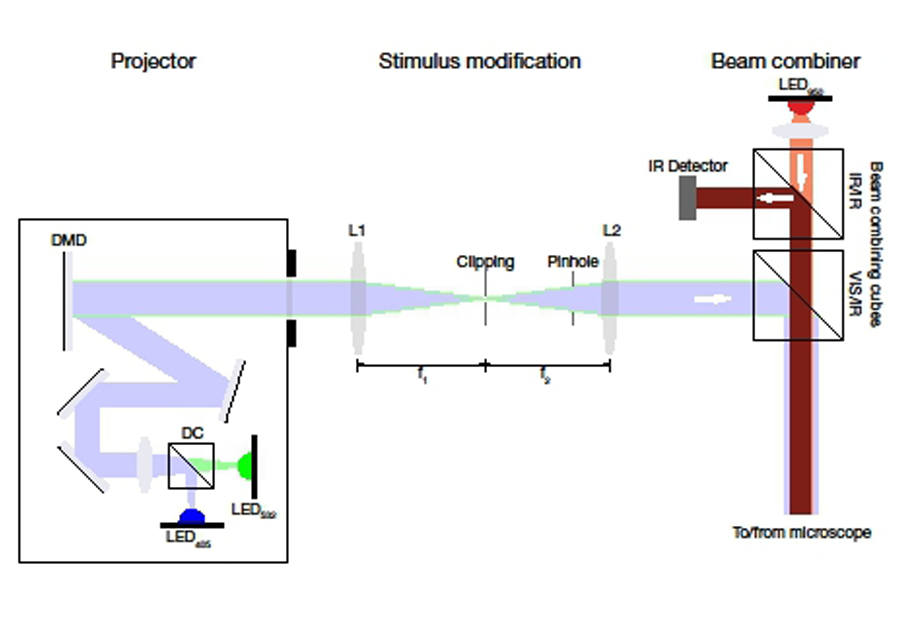
Figure 1: Projection optics. Light emitted by the LEDs is combined by a dichroic mirror (DC), collimated and projected onto the digital micromirror device (DMD). The image generated by the DMD is projected through the L1-L2 lens system, containing clipping mask and pinhole if required. In the VIS/IR beam combining cube, the visual stimulus is reflected towards the microscope and combined with the IR illumination from the transillumination LED. In the IR/IR beam combining cube, the IR light from the LED is passed towards the microscope, while the IR light from the laser coming from the sample is reflected onto the IR detector for IR-Dodt contrast.
All projectors using LED illumination can output light of precisely defined wavelengths (determined by LED and clean-up filter selection). In addition, compared with regular (LCD-based) projectors, DMD-based devices such as the LightCrafter have the advantage of high switching speeds. In these devices, the frame rate is directly linked to the greyscale bit depth, allowing a trade-off between frame rate and bit depth. In the case of the LightCrafter, the range is from 120 Hz frame rate at 8-bits to 4 kHz at 1-bit. This is where the Stage VSS software shows its strengths, as it has been written to leverage the flexibility afforded by the LightCrafter hardware.
Once the stimulation pattern has been generated and is projected, the next step is getting the stimulus to the tissue. For this, the projection needs to be coupled into the microscope.
Through the condenser
Coupling the visual stimulus into the condenser is achieved through the transillumination port at the base of the SliceScope10. To avoid blocking access to other optical components that typically make use of this port, a beam combiner is used (Figures 1 and 2A). This combiner enables the visual stimulus projection to be coupled to the transillumination port while still providing space for transillumination components (e.g. LEDs).
The simplest approach is to mount the projector to a miniature optical rail system (e.g. LINOS Microbench, Qioptiq or Cage System, Thorlabs) which is linked to the beam combiner. This rail system allows additional elements to be mounted along the optical axis of the projector. This provides a very convenient means of adapting the projected stimulus to meet the experimental requirements, as described in the following paragraphs.
A neutral density filter wheel (motorised to allow remote control, Figure 2B) is added to the beam path to control the brightness of the visual stimulus. The size of the projection (i.e. how large a projected pixel is on the retina) is controlled by a set of two lenses (Figure 1). The minification of the projection is determined by the ratio of the focal length of these lenses. If clipping of the stimulus is required, a clipping mask (iris diaphragm) can be placed in the image plane between these two lenses.
Final focusing onto the sample is achieved by moving the condenser in the z-axis. Closing down the field stop located on microscope will relay the exact z-plane in which the projector is focused. The projected image should be focused on the photoreceptor layer of the retina before experimentation.
As condensers are not designed to be achromatic, the focal planes of the different used stimulation colour channels will show a substantial shift when projecting through the condenser. To compensate for this, a pinhole can be positioned at the conjugate plane to extend the depth of focus of the projector. In addition to reducing effects of chromatic aberration, this extended focus depth simplifies focal alignment, by increasing the tolerance.
The required components for coupling combined visual stimulation and near-IR transillumination into Scientifica SliceScopes are available from Scientifica and can be configured to the user’s requirements.
Scientifica's SliceScope
A stable and compact upright microscope designed to meet the demands of complex electrophysiology studies and related techniques - including two-photon imaging and optogenetics.
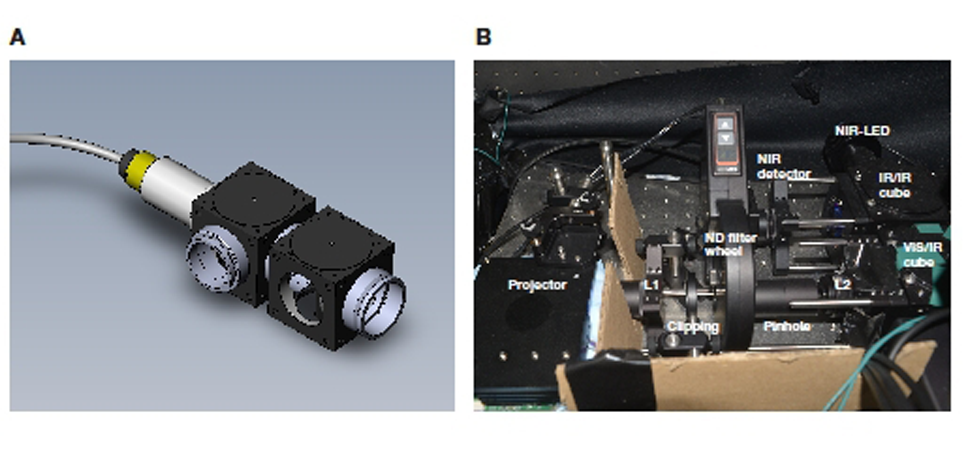
Figure 2: Through the objective configuration. A: 3D rendering of the double beam combining cube with IR LED connected at the left. B: Complete stimulation system as installed in the Schwartz Lab.
Through the objective
Coupling the projected visual stimulus through the objective need not be more complicated than through the condenser. Indeed, the most straightforward approach is analogous to the configuration discussed above: coupling the stimulus through an existing epi-illumination port. These ports are generally used for bringing excitation light to the sample in widefield fluorescence imaging. As above, a miniature rail system is used to line up the projector and required optical elements (e.g. lenses and filter wheel). An adapter interfacing the rail system with the epiillumination connector is all that is the needed to make the mechanical connection to the microscope. A filter cube equipped with a mirror (dichroic, regular or 50/50) is positioned in the light path using the filter revolver of the epi-fluorescence module. The advantage of having a 50/50 mirror in the filter turret is that it is possible to image the reflection of the stimulus with a camera through the mirror, using a reflective surface at the sample plane.
Similar to above, the stimulus can be focused on the sample using the objective after adjusting the position of the minification lenses to bring the projected image onto the focal plane of the objective. As good objectives are achromatically corrected, it is not necessary to expand the depth of focus in this configuration, eliminating the need for a pinhole.
When imaging, the microscope is switched to multiphoton mode. Once a recording from the target cell is established, the objective is adjusted so that the stimulus is focused onto the photoreceptors and the microscope is carefully switched into epifluorescence mode.
The components required for coupling the visual stimulation into Scientifica SliceScopes are available from many suppliers of optomechanics.
General considerations
Synchronisation of visual stimulation and the corresponding electrophysiological recordings can be achieved using a photodiode to detect the stimulus timing. For this, a small square in one of the projection corners can be used to display a timing signal (e.g. a brief flash) to indicate defined time points in the experiment (stimulation start, change of stimulus, etc.). Splitting the output from the stimulation PC to a separate monitor as well as the projector makes it possible to place a photodiode on the monitor in the ‘timing corner’ and used the output of this diode as a synchronisation signal. To prevent the timing signal from interfering with the actual stimulation, this corner of the projection can be clipped using an iris diaphragm clipping mask placed between the two minification lenses (see above).
Both of the configurations discussed above are aimed at sequentially projecting a visual stimulus and recording electrophysiological data. For the majority of current applications, this will not be a limitation. If simultaneous functional imaging (e.g. Ca2+) and visual stimulation are required, a different approach will be necessary.
The most straightforward manner to get an existing system setup for simultaneous imaging and stimulation will be a variant of the ‘through the condenser’ configuration discussed above. In this case, imaging would be performed through the objective, while stimulation is applied through the condenser. To avoid contamination of the imaged signal by the visual stimulus, careful planning of the experiment is needed. The wavelength bandwidth of the projector light source and the excitation and emission bands of the used fluorophores would need to be taken into consideration. But, by varying the dichroic mirrors and optical filters, or presenting visual stimuli temporally interleaved with detection by the photomultipliers, it is possible to minimise cross-contamination.
The through-the-objective configuration discussed above can also be adapted for simultaneous imaging and stimulation. While that comes at the price of a change of some of the optical elements at the microscope’s core, it will allow the substage detection of fluorescence to be used during stimulation, with its higher photon collection efficiency.
Conclusions
In this technical note, we have presented two approaches to projecting visual stimuli on the ex vivo retina in a standard two-photon microscope. We show that the required equipment and software is readily available and that the integration is straightforward. Importantly, it is possible to add such a stimulation system to an existing multiphoton microscope without any significant changes to the microscope itself.
It is worth noting that, similar to most fields, visual neuroscience benefits greatly from standardisation in visual stimuli, as this leads to results that are more comparable across labs. Therefore, widespread adoption of similar projector systems has the potential to push the field forward.
References
1. Masland, R. H. The Neuronal Organization of the Retina. Neuron 76, 266–280 (2012).
2. Baden, T. et al. The functional diversity of retinal ganglion cells in the mouse. Nature 529, 345–50 (2016).
3. Helmstaedter, M. et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174 (2013).
4. Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science (80-. ). 246, 73–76 (1990).
5. Euler, T. et al. Eyecup scope-optical recordings of light stimulus-evoked fluorescence signals in the retina. Pflugers Arch. Eur. J. Physiol. 457, 1393–1414 (2009).
6. Margrie, T. W. et al. Targeted whole-cell recordings in the mammalian brain in vivo. Neuron 39, 911–918 (2003).
7. Wei, W., Elstrott, J. & Feller, M. B. Two-photon targeted recording of GFP-expressing neurons for light responses and live-cell imaging in the mouse retina. Nat. Protoc. 5, 1347–52 (2010).
8. Jacobs, G. H., Neitz, J. & Deegan, J. F. Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature 353, 655–656 (1991).
9. Nikonov, S. S., Kholodenko, R., Lem, J. & Pugh, E. N. J. Physiological Features of the S- and M-cone Photoreceptors of Wild-type Mice from Single-cell Recordings. J. Gen. Physiol. 127, 191–204 (2006).
10. Jacoby, J., Zhu, Y., DeVries, S. H. & Schwartz, G. W. An Amacrine Cell Circuit for Signaling Steady Illumination in the Retina. Cell Rep. 13, 2663–70 (2015).
Other SciMethods you may be interested in...
SciMethods: Simultaneously recording from four neurons using PatchStar and MicroStar manipulators
SciMethods: Paired Blind Polytrode and Juxtacellular Recordings In Vivo
SciMethods: Using a Scientifica Multiphoton SliceScope for Fluorescence Lifetime Imaging
SciMethods: Controlling and Coordinating Experiments in Neurophysiology


)