
Electrophysiology protocols for LTP and LTD: Recording synaptic plasticity with whole-cell patch clamp
Learn how to record long-term potentiation (LTP) and long-term depression (LTD) using whole-cell patch clamp. Step-by-step guide to measuring synaptic plasticity in individual neurons.

by Emily Winson-Bushby, PhD Student, Grubb Lab, Centre for Developmental Neurobiology
Long-term potentiation (LTP), first discovered in hippocampal neurons by Bliss, Lømo and Gardner-Medwin 1,2, has been described by many as the canonical form of synaptic plasticity. Long-term depression (LTD), its logical partner, was identified in the following two decades – first in the cerebellum3, and then in the hippocampus 4. The basic principle of both is this: synapses are able to adjust their strength in response to the magnitude of inputs they receive, shaping network dynamics and storing information – and as such they represent a type of learning that occurs at the level of the synapse. In LTP, when high-frequency stimulation is applied to presynaptic cells, their postsynaptic partners show an elevation in amplitude of excitatory postsynaptic potentials (EPSPs)1. In LTD, application of low-frequency stimulation triggers a decrease in amplitude of EPSPs4. Under certain experimental conditions, LTP and LTD can be stimulated in the same cell sequentially5.
Often, LTP/LTD experiments are performed using extracellular field recordings6, which are easier to maintain for a prolonged period than a patch. However, field recordings output fEPSPs (field EPSPs), which represent the summed synaptic activity experienced by many cells. If you want to record LTP/LTD within an isolated cell type occurring within heterogeneous tissue, you will need whole-cell patch clamp, which allows you to record synaptic events from within specific individual cells.
Trying to record LTP or LTD from acute slices in the whole-cell patch configuration? Here’s your guide.
Basic setup
For an LTP/LTD experiment, you need two things: 1) the ability to record synaptic events for a sustained period, and 2) the ability to provide presynaptic stimulation. Most use electrical stimulation for the latter, but more recently, alternative methods have begun to be used – for example, optogenetic stimulation of presynaptic cells7. This article will consider electrical stimulation, using a simple stim electrode.
The format of an LTP/LTD experiment is as follows: first, a baseline recording period to test amplitude of synaptic events; next, a brief plasticity induction protocol; and finally, a post-induction recording period to measure whether synaptic strength has changed, and potentially for how long the change persists. Typically, a 30-minute recording will be long enough for this, and once you’ve developed the ability to maintain a stable patch for this duration, you’re set! The experiment should be run in two strands – 1) a plasticity induction arm as described, and 2) a control arm, in which you replace the plasticity-inducing stimulus with a standard synaptic recording, or alternatively leave the time empty.
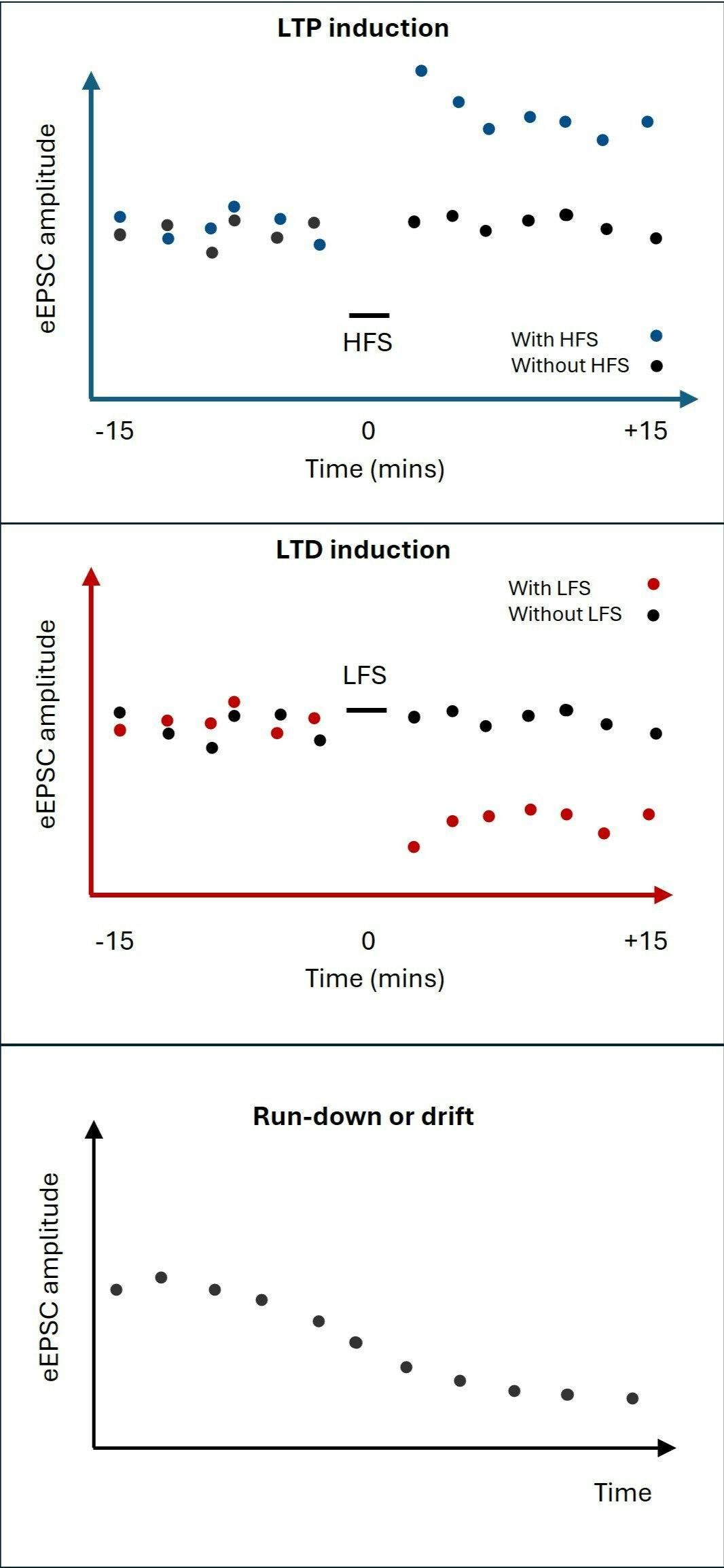
Representations of results traces which may be obtained from LTP (upper) or LTD (middle) experiments, indicating changes in eEPSC amplitude following induction protocols (HFS or LFS), as compared to control arms (without HFS or LFS). In some experiments eEPSC amplitude may shrink with time without any induction protocol, as a result of run-down or drift (lower panel).
Recording synaptic events
When patching, it is most straightforward to record synaptic events in voltage clamp, meaning that they are recorded not as EPSPs, but as excitatory postsynaptic currents (EPSCs). You can record EPSCs under voltage clamp at -60mV, using a K+-gluconate internal, or for a better space-clamp use a Cs+-gluconate internal and clamp the voltage at -70mV. In both configurations, EPSCs will register as inward or negative-going events with the classic shape of rapid onset and slow decay time. Note that some plasticity protocols rely on a post-synaptic action potential - in these cases, potassium based internal solutions must be used8, as a caesium-based internal will alter AP waveforms and impede high-frequency firing. In addition, the cell should be help in current clamp mode instead of voltage clamp, for either the full experiment or the induction period, to allow APs to alter the membrane potential and initiate downstream effectors.
There are two options as to how you go about recording synaptic events – spontaneous, or evoked. The most straightforward option is to record spontaneous synaptic events (spontaneous EPSCs, aka sEPSCs), particularly if you know that the vast majority of inputs received by your cell will be from your presynaptic partner cell of interest. The more reliable option, however, is to use evoked excitatory postsynaptic currents (eEPSCs), as in this case you know the provenance of the events you evoke. In the olfactory bulb, for example, an effective way to do this is to place your extracellular stim electrode on axon bundles in the olfactory nerve layer – this way you know that the events you evoke come from the olfactory sensory neuron axons you stimulate. Typically, you will already need a stim electrode placed for the LTP/LTD induction itself, so this option should require little extra work, and in the absence of other counterindications is perhaps therefore a no-brainer. Note that eEPSCs, like fEPSPs, are also summed events, but they are a composite of many inputs to the same cell, whereas the latter is the composite of many inputs into different cells. A benefit of using eEPSCs is that they are larger than sEPSCs, and therefore less likely to be obscured by noise. If using eEPSCs, use the same intensity for every test-stim throughout the recording duration, at least on a within-cell basis.
Record eEPSCs at consistent intervals of 60s to allow the synapses to recover between stims and to reduce the likelihood of inducing any unintended plasticity. Make sure that the pulse from your stim electrode is very brief, so as to not obscure the recorded event, and the monosynaptic EPSC should occur after a very brief latency (<10ms). Repeat for approx. 15-minutes to establish a clear baseline.
A good practice is to play around before initiating your actual recording with the amplitude of the stim so that you get a moderately sized EPSC to begin within (perhaps around 500pA), to give it room to either grow or shrink according to your induction protocol. This means that individual cells receive different nominal stimulation intensities, but intensity remains consistent within-cell, once the actual recording starts. A more in-depth method for doing this is to map a basic input-output (I-O) curve for your cell, so that you can identify the stim intensity at which the EPSC amplitude reaches its maximum. For LTD, it is clear that you need a starting size of EPSC that is large enough to allow for shrinkage with plasticity. However, it has been argued that the reverse is not true for LTP9. At first thought, it seems clear that choosing a test-stim intensity in the middle of the I-O dynamic range curve allows space for growth. However, movement up along the I-O curve in this context may reflect better the number of axon bundles being stimulated rather than the strength at which individual synapses are stimulated. Thus, picking a higher test-stim intensity may mean more axon bundles stimulated, and more synapses participating in the recording which have potential to undergo LTP, resulting in a larger and clearer result.
Lastly, when using eEPSCs, it’s very important to confirm that your stim electrode is stimulating the presynaptic axons, but not
stimulating the postsynaptic cells directly. This way you can be sure that the events you measure are entirely synaptic in nature. This can be done easily by performing some test recordings in the presence of TTX, which, if no direct stimulation is present, should totally abolish the eEPSC (by inhibiting transmission in the presynaptic axon). It is a good practice to establish a working distance between the stim electrode and postsynaptic dendrites (in olfactory bulb this is the distance between the stim electrode and glomerulus), validate this distance, and then stick to it.
Plasticity induction
To induce plasticity, you need a reliable stimulation protocol, and different cell types respond well to different types of stimulus. It’s a good idea to look in the literature to see if there are pre-existing examples of certain protocols working in your cell type of interest. Commonly-used protocols for LTP include high-frequency stimulation (HFS, also known as tetanus), which usually refers to sustained 50-100Hz stimulation, or theta-burst stimulation (TBS), referring to high-frequency stimulation applied in bursts instead of continuously. LTD is typically induced using low-frequency stimulation (LFS), referring usually to 1-5Hz stimulation. Apply the stimulus to the presynaptic axons or cells using the extracellular stim electrode. Be sure at this point to replace your test stim with the plasticity-inducing stim instead of applying them immediately one after the other!
After applying the stim, you’ll want to continue recording eEPSCs at 60s intervals, if possible for another 15 minutes. If your induction has been successful, you’ll see a marked increase or decrease in eEPSC amplitude if LTP or LTD have occurred, respectively.
Ultra-low noise, highly stable equipment built for precision patch clamping
Sustaining a long patch – the necessity of a control arm
Sustaining a patch for 30 minutes is somewhat of a big ask – maintaining a prolonged good-quality patch with a low enough series resistance to pass inclusion criteria is difficult, and in this experiment you’ll also want series resistance to stay consistent not just across individual recordings, but across the entire panel of test stims that you apply to the cell. Make sure that your protocol does regular membrane tests – if possible run them between each test stim, and exclude any recordings which do not pass your criteria for series resistance size (typically Rs<30MΩ) and consistency (ΔRs<20%) across the entire sequence of test stims.
To give yourself the best chance of maintaining the patch, make sure that pipette drift and vibration are minimised on your rig, and track the position of your pipette through the recording by marking its initial position on your screen with a whiteboard marker or piece of blu-tac. To support cell health throughout long patches, make sure carbogenation of your ACSF is working well, and that ACSF is circulating effectively to provide your neurons with oxygen and glucose. Make sure to keep your internal on ice, to avoid degradation of the ATP and GTP it will provide to your cells.
Even if you are maintaining your patch perfectly, in some circumstances, patched neurons will experience run-down10 in synaptic responses anyway (see diagram below), as a result of the strained nature of a prolonged recording setup. It is for this reason that it is highly important that you do not only perform a plasticity induction arm for your experiment, but also a control arm. This is important in particular for LTD experiments, in which the effect you are looking for is a reduction in synaptic amplitude. However, this is an important consideration in LTP experiments also, as run-down may obscure ongoing LTP.
The duration of your baseline period may also need to be adapted depending on the form of plasticity you are inducing. Some LTP/LTD mechanisms are strongly dependent on secondary messengers and intracellular kinases which can be ‘washed out’ in the whole-cell patch clamp configuration11. In these cases, it is crucial to keep the baseline period as short as possible (whilst still ensuring you have a stable response) or the perforated patch-clamp method can be used which maintains the intracellular contents whilst giving you electrical access to the cell.
Drift of the stim electrode can also be an issue, resulting in a superficially similar run-down of synaptic events – if the electrode gradually drifts away from the nerve bundle with time, synaptic response size may also diminish. Test the stability of the stim electrode by monitoring it against a whiteboard marker or blu-tac reference point as above, over a 30-minute period. It is also a good idea to confirm that your stim box applies pulses of consistent size when repeated across a 30-minute period. You can test this in a slice-free set-up by applying pulses to the bath and measuring the size of the stim artefact received by a recording pipette positioned free in the bath.
Good luck!
If you keep in mind all of the above, with luck, your experiment may well be successful! For more information on LTP and the methodologies that have historically been used to investigate it, see these amazing reviews by Jiang and colleagues12, and Glasgow and colleagues13. Best of luck!
References
1. Bliss, T. V. P. & Lømo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232, 331–356 (1973).
2. Bliss, T. V. P. & Gardner-Medwin, A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology 232, 357–374 (1973).
3. Ito, M., Sakurai, M. & Tongroach, P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. The Journal of Physiology 324, 113–134 (1982).
4. Dudek, S. M. & Bear, M. F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A 89, 4363–4367 (1992).
5. Dudek, S. & Bear, M. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci 13, 2910–2918 (1993).
6. Abrahamsson, T., Lalanne, T., Watt, A. J. & Sjöström, P. J. Long-Term Potentiation by Theta-Burst Stimulation using Extracellular Field Potential Recordings in Acute Hippocampal Slices. Cold Spring Harbor protocols 2016, pdb.prot091298 (2016).
7. Nabavi, S. et al. Engineering a memory with LTD and LTP. Nature 511, 348–352 (2014).
8. Chen, H.-X., Otmakhov, N. & Lisman, J. Requirements for LTP Induction by Pairing in Hippocampal CA1 Pyramidal Cells. Journal of Neurophysiology 82, 526–532 (1999).
9. Daniel V Madison. Can someone advise on the whole-cell LTP problem? ResearchGate https://www.researchgate.net/p....
10. Kato, K., Clifford, D. B. & Zorumski, C. F. Long-term potentiation during whole-cell recording in rat hippocampal slices. Neuroscience 53, 39–47 (1993).
11. Malinow, R. & Tsien, R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature 346, 177–180 (1990).
12. Jiang, F. et al. Advances in the Electrophysiological Recordings of Long-Term Potentiation. Int J Mol Sci 24, 7134 (2023).
13. Glasgow, S. D., McPhedrain, R., Madranges, J. F., Kennedy, T. E. & Ruthazer, E. S. Approaches and Limitations in the Investigation of Synaptic Transmission and Plasticity. Front. Synaptic Neurosci. 11, (2019).
About the author
Emily Winson-Bushby is currently a PhD student in the lab of Prof Matthew Grubb, at the Centre for Developmental Neurobiology in London. She studied Natural Sciences at the University of Cambridge, followed by an MRes at the University of Edinburgh, and now works on responses of circuits to regeneration in the olfactory system.

Emily Winson-Bushby, Centre for Developmental Neurobiology, KCL










)