Scientifica's top 10 neuroscience stories from July 2021
Included in July's top neuroscience stories is the most comprehensive map of the 3D shapes of neurons and their synapses to-date, a helmet that uses a magnetic field to noninvasively shrink glioblastoma tumours, and a new optogenetic tool that uses x-rays to control cells deep in the brain.
1. Scientists rejuvenate mouse brains with ketamine or flickering light
Researchers at the Institute of Science and Technology (IST) Austria have discovered two new methods for increasing plasticity of adult mouse brains, enabling them to reorganise neuronal connections like they can during development.
The two methods involve using either repeated ketamine anaesthesia or non-invasive 60 Hz light flickering to remove the perineuronal net, restoring the adaptability seen in young brains, where the brain reorganises neuronal connections much more freely than in adult brains.
The perineuronal net, an extracellular structure that envelops certain neurons, stabilising synaptic connections between them and preventing new ones from forming, was the target of the two techniques. Both techniques are minimally invasive, and work by stimulating microglia to breakdown the perineuronal net. Once the net is removed, neurons are more sensitive to new inputs and new synapses can be formed, increasing the adaptability of the brain.
Due to the non-invasiveness of the treatments, there is potential for their use in humans to treat a range of disorders. However, there are risks of increasing the adaptability of the brain, for example, if something traumatic happens when the net is reduced. The researchers will continue investigating with the aim of fully understanding the molecular mechanisms behind the treatments, as well as their applications.
Restoring the brain's adaptability
2. Researchers prove powerhouse malfunction as the major cause of Parkinson’s disease
Scientists at the University of Copenhagen have discovered that dysregulation of immune system genes, which are essential for brain functions as well as fighting viruses, is a major cause of 90-95% of cases of Parkinson’s disease.
The study showed that this dysregulation means that mitochondria cannot get cleaned up after being damaged, leading to an accumulation of damaged mitochondria that cannot function correctly. The dysfunctional mitochondria cause neurons to have insufficient energy to stay alive, leading to their gradual death.
It was discovered that the dysregulated pathway is that of type 1 interferon, which is negatively regulated by the PIAS2 protein. In normal circumstances, the PIAS2 protein blocks the type 1 interferon pathway. However, when an infection is present, the PIAS2 protein unblocks the pathway, enabling infections, damaged proteins and mitochondrial waste to be cleared. High accumulation of the PIAS2 protein was found to be responsible for blocking the pathway in Parkinson’s disease, causing damaged proteins and mitochondrial waste to build up.
The death of neurons is what causes symptoms of Parkinson’s disease. It is hoped that results from this study will lead to further research into ways to counteract the blocked pathway, in order to treat and prevent the disease.
Malfunctioning mitochondria
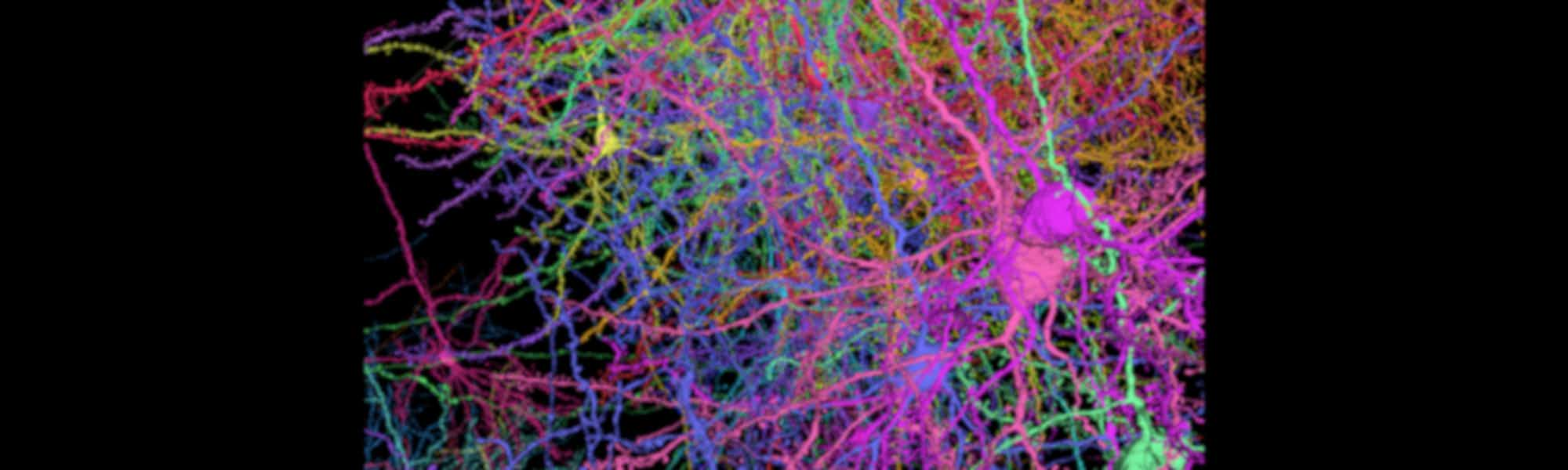
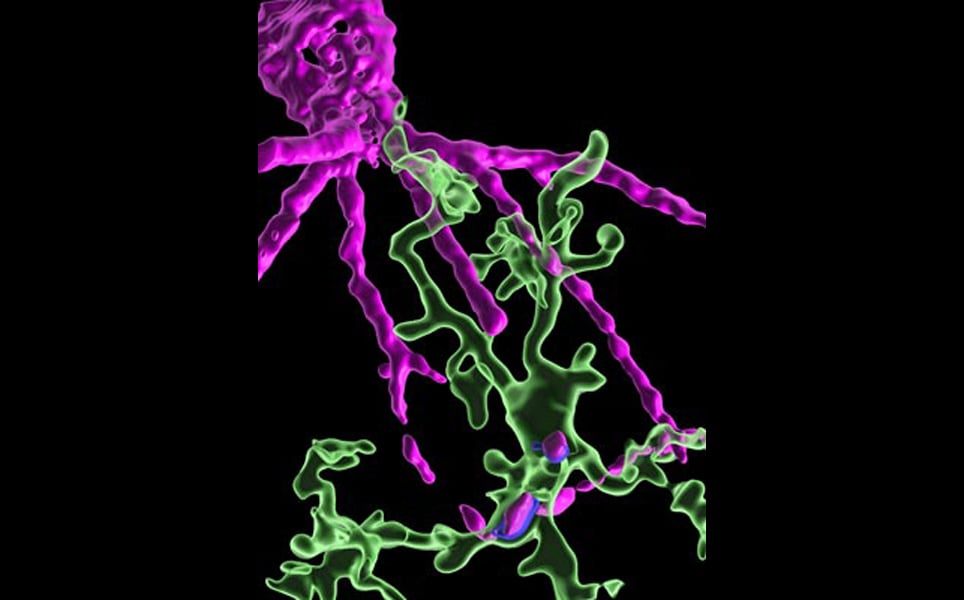
3. Detailed map captures 3D shapes of neurons and their synapses in stunning detail
A comprehensive 3D map of the 200,000 cells and almost 500 million synapses in a tiny section of a mouse brain has been created by researchers at the Allen Institute, Princeton University and Baylor College of Medicine.
The detailed map of all the structures and connections in a cubic millimetre section of the visual neocortex, which process what a mouse sees, was created as part of the the Machine Intelligence from Cortical Networks (MICrONS) program. It took five years to complete, with the goal improving machine learning.
This publicly available data contains the most cells and connections of any dataset of this type, capturing entire local circuits and 3D shapes of individual neurons. It will be valuable to neuroscientists and researchers investigating how the brain transmits information along defined circuits, and treatments for brain disorders where these connections are altered.
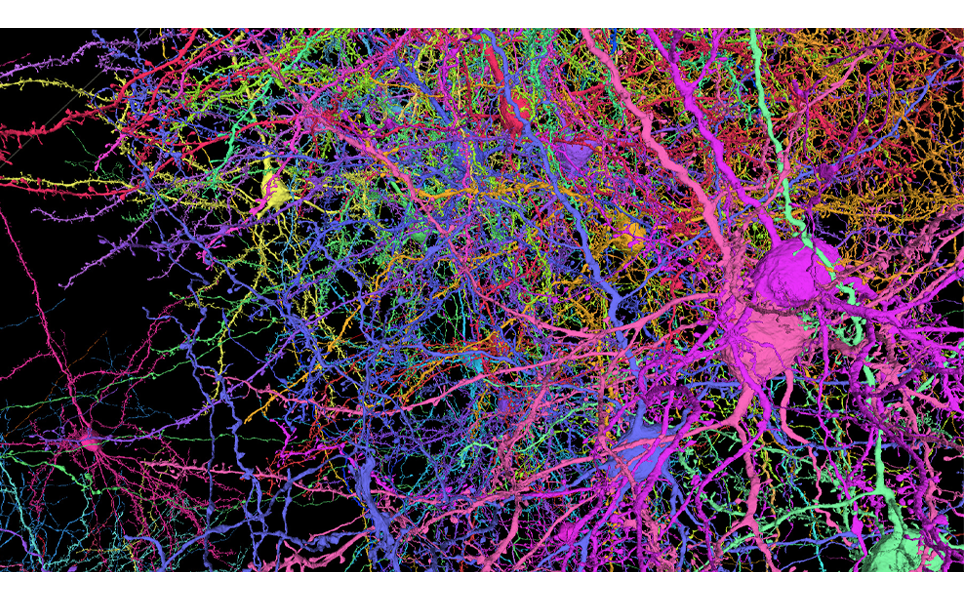
See the brain in more detail
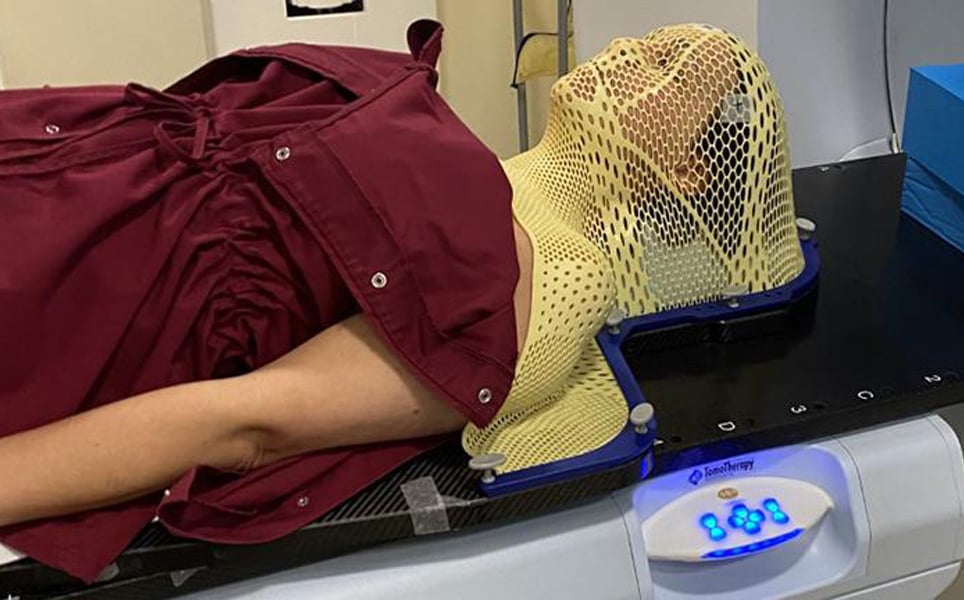
4. Investigational magnetic device shrinks glioblastoma in first-in-world human test
Using a helmet that generates a non-invasive oscillating magnetic field, scientists at Houston Methodist Neurological Institute have succeeded in shrinking a deadly glioblastoma tumour by more than a third.
The helmet enables the therapy to be administered by the patient in their own home. During the five weeks of treatment, the magnetic therapy was well-tolerated and tumour mass and volume reduced.
Although the patient sadly died from an unrelated injury a month into treatment, the results provide possibilities for future non-invasive and non-toxic treatments for brain cancer.
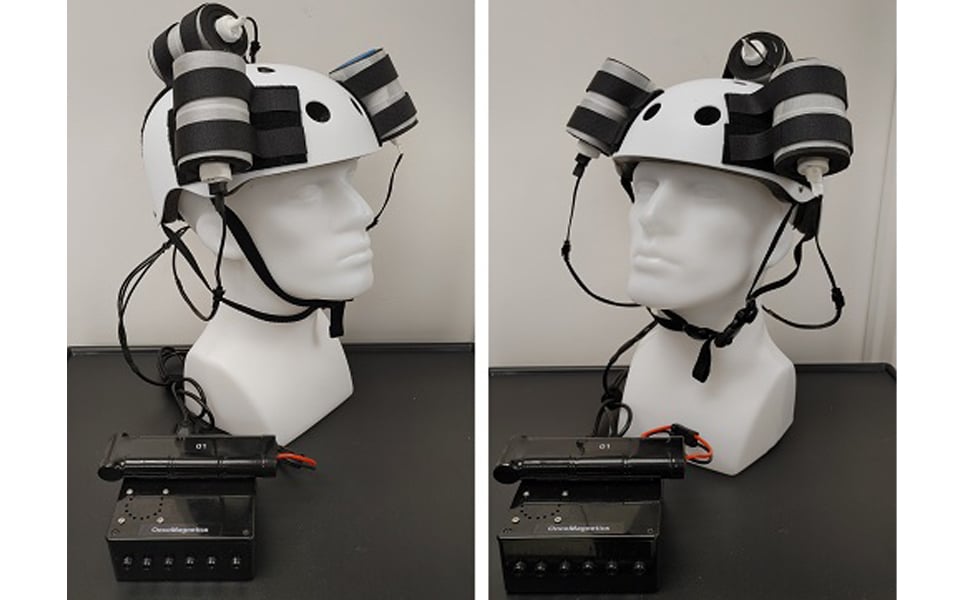
The future of brain cancer treatments?
5. Neuroprosthesis restores words to man with paralysis
A ‘speech neuroprosthesis’ developed by researchers at UC San Francisco, which translates signals transmitted from the brain to vocal tract into words, has successfully enabled a man with severe paralysis to communicate in sentences.
By translating signals, meant to control muscles of the vocal system for speaking words, rather than those designed to move the arm or hand when typing, more rapid and natural speech can be achieved. Using brain recordings from epilepsy patients, models and methods of real-time decoding of patterns of brain activity were developed.
These were then tested in a paralysed patient, who had suffered a brainstem stroke that severely damaged the connection between his brain and rest of the body, including the vocal tract. A high-density electrode array was implanted over the patient’s speech motor cortex, enabling neural activity to be recorded. A 50-word vocabulary was created that advanced computer algorithms could detect from brain activity. These could then be used to create hundreds of different sentences relevant to the patient’s daily life.
The system was able to decode words from brain activity at a rate of up to 18 words per minute, with 93% accuracy. This is the first time that a neuroprosthesis has successfully decoded brain signals into complete words, and provides promise for restoring the ability to communicate in those who have lost the ability due to factors such as a stroke or an accident.
Translating brain signals into sentences
6. Brain ‘noise' keeps nerve connections young
Researchers at EPFL have discovered that a form of neuronal communication that was previously thought to be background noise is actually required to maintain synapses as animals age.
In normal neuron-to-neuron communication, electrical signals are transmitted across a neuron to a synapse, where they stimulate the release of neurotransmitters, which enable the electrical signal to be transmitted to the next neuron. This is evoked neurotransmission. However, sometimes, neurotransmitters are released at the synapse in the absence of an electrical signal. These mini events have long been thought of as background noise.
In 2014, it was discovered that ‘minis’ have an important role in the development of synapses. Now, in fruit fly studies, scientists have shown that inhibiting both evoked and miniature neurotransmission caused synapses to age prematurely. However, increasing evoked neurotransmission caused no change to the ageing synapses, whereas increasing the frequency of the mini events kept synapses intact. Increasing these events also caused middle aged flies to have motor ability comparable to young flies.
As defects in miniature neurotransmission has been linked to a range of neurodevelopmental disorders in children, understanding how this neurotransmission affects synapses and behaviour could help better understand and treat these disorders.
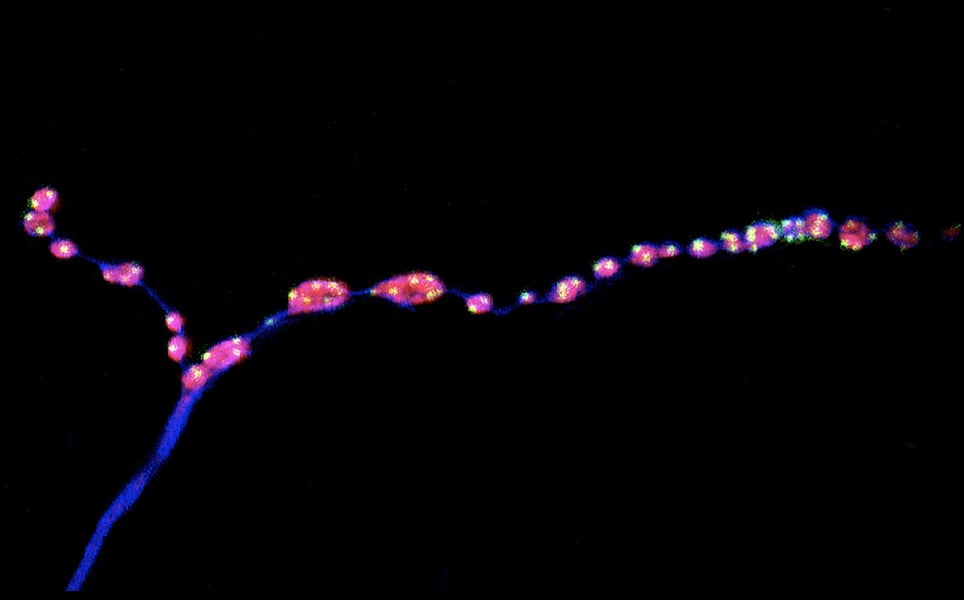
Mini neurotransmission, large impact
7. Scientists can detect brain tumours using a simple urine or blood plasma test
For the first time, two tests that can detect glioma, a type of brain tumour, from the blood plasma or urine of patients, have been developed by scientists at the Cancer Research UK Cambridge Institute.
It is hoped that these tests could be used in the future by GPs to monitor patients at high risk of brain tumours; those who have had a brain tumour removed. This could be more convenient and faster than the current method of having an MRI scan every three months, or these tests could even be used between MRI scans, helping returning brain tumours be detected earlier, and reducing anxiety of patients in between scans.
Improving detection of glioma
8. Memory making involves extensive DNA breaking
A new study has shown that when quickly creating memories about a dangerous experience, neurons and brain cells break their DNA in more locations that previously thought. This is to provide quick access to the genetic instructions for memory storage.
These DNA double-strand breaks (DSBs) occur in multiple key brain regions, and they could be of concern, as although they are repaired, the repair process could be compromised with age. DSBs that are not repaired have been associated with neurodegeneration and cognitive decline.
The studies in mice demonstrated that creating fear memories doubled the number of DSBs in neurons in the hippocampus and prefrontal cortex. Of the 300 genes affected, 206 were common to both regions, many being associated with synaptic function. Measurements of RNA showed that increased DSBs correlated with increased transcription and expression of the affected genes.
The team also discovered that glia also showed changes in the expression of hundreds of genes after fear conditioning, especially in genes involved in the glial response to hormones. They found that many DSBs in glia occur at genomic sites related to glucocorticoid receptors, which made sense as glucocorticoids are secreted in response to stress.
Further research will investigate how much of a threat to later brain health the DSBs are.
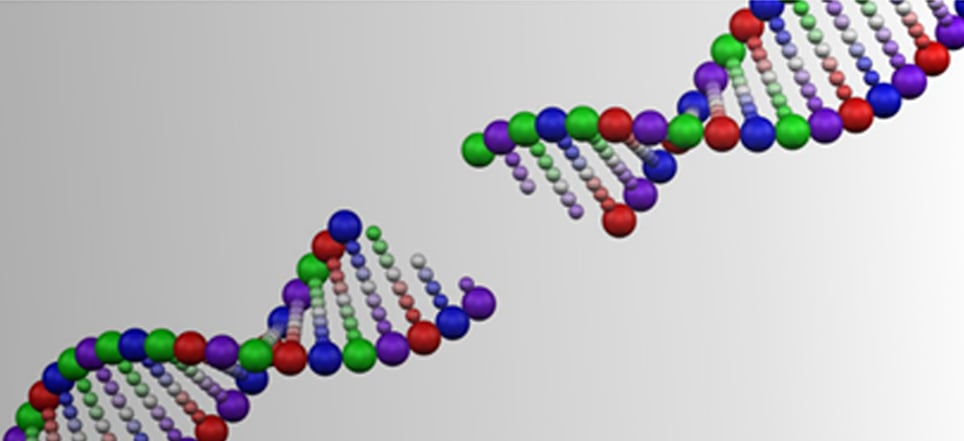
Potential danger of DSBs
9. Spinal fluid biomarkers detect neurodegeneration in living patients
A combination of biomarkers in the cerebrospinal fluid (CSF) of living patients can be used to detect Alzheimer’s disease and other forms of neurodegeneration, scientists at University of Pennsylvania have found.
Currently, Alzheimer’s disease and related diseases can only be confirmed through autopsy of a deceased patient’s brain. The development of biomarkers would enable accurate disease diagnosis when the patient is still alive.
In 2018, a new framework suggested that a combination of three Alzheimer’s disease biomarkers in the CSF – pathologic amyloid plaques (A), tangles (T), and neurodegeneration (N), collectively called ATN, could detect the disease. However, this new research proposes that the ATN framework can be extended to also detect frontotemporal degeneration.
Distinguishing frontotemporal degeneration from Alzheimer’s disease can be difficult, as symptoms often overlap. The findings from this research suggest that adding neurofilament light chain (NfL) to the ATN framework would enable more accurate diagnosis of frontotemporal degeneration.

More accurate diagnosis of neurodegeneration
10. New optogenetic technology that remotely controls proteins in the deep brain
In a collaborative study by scientists at Fujita Medical University School of Medicine and Nara Institute of Science and Technology, a new non-invasive method of remotely controlling nerve cells in the deep brain without damaging tissue has been developed.
Using a scintillator, a molecule that converts x-rays into visible light, optogenetics was used to remotely control specific cranial nerve functions. The opsin used in this research is sensitive to light wavelengths in the visible region, but visible light is unable to penetrate living tissue. X-rays, however, are able to penetrate the body. Using a scintillator particle to convert x-rays into yellow light once they have penetrated the body, means the opsin expressed in a specific region of the mouse brain can be activated noninvasively, without needing to implant optical fibres.
The researchers demonstrated that the scintillator particle injection didn’t cause inflammation, with low doses being sufficient to activate opsins. This technology could enable treatments to be developed that can be delivered to target sites more efficiently, reducing side-effects.
Utilising x-rays for optogenetics
Banner image credit: Allen Institute for Brain Science

)