
Scientifica's top neuroscience stories from November 2021
In this article, we explore some of the exciting neuroscience stories that have emerged from November. These include, an injectable therapy that harnesses “dancing molecules” to reverse paralysis and repair tissue after severe spinal cord injuries, identification of novel brain signatures that are unique to the response of each antidepressant and insight into how the brain balances fear.
Ever been lost in the grocery store?
New research led by Li Zheng, a postdoctoral fellow in the Ekstrom's lab at the University of Arizona, could bring scientists a step closer to understanding how the brain remembers spatial environments, particularly those that are alike and how the brain avoids confusion or doesn't. This is an insight that researchers have long struggled with.
The study's 27 participants were asked to memorise an animated video from the perspective of someone walking around three, nearly identical, virtual cities. Each city contained stores, and although all stores were in the same place, not every city had the same stores. Considering how similar the cities were, participants found it challenging to answer questions about their layout.
Brain patterns observed by the researchers were often similar to one another. However, when participants were asked about stores that emerged in more than one city, their brain activity was unexpectedly different. The findings indicate that that the brain distinguishes very similar environments, such as two stores from the same supermarket chain, as if they are even more different than two places that are nothing alike.
Their research could ultimately help to provide better insight into why conditions such as stroke and Alzheimer's disease cause symptoms such as disorientation and poor spatial memory.
Read the full story here

UTSW-led research identifies new imaging biomarkers that predict antidepressant response
Combining neuroimaging and artificial intelligence, a research team led by the University of Texas Southwestern Medical Center, has identified novel brain signatures unique to the response of each antidepressant.
As well as using pre-existing data, researchers tested the antidepressant drug sertraline with a control group taking a placebo. Those who did not respond to sertraline after 8 weeks received the antidepressant bupropion. In over 300 participants, non-invasive functional magnetic resonance imaging (fMRI) was carried out to assess changes in brain function at rest, as well as during the reward task.
Using that data and new advances, researchers built new machine learning models that demonstrate to scientists and clinicians, which particular brain regions and circuits are linked with prediction of treatment response to each medication.
Dr. Madhukar Trivedi, M.D., Professor of Clinical Psychiatry, and Director of the Center for Depression Research and Clinical Care, said the new biomarkers could spare patients suffering severe depression two to three months of taking the wrong medication.
Read the full story here
Brain connections have their own tempo
By being able to categorise and associate the stimuli it obtains from our five senses, the cerebral cortex connects this information together to make sense of it. In order to achieve this, during embryonic development and early postnatal life, different types of neurons form cortical connections.
A question that arises from this process is what is the biological mechanism by which this delicate assembly is created? A research team at the University of Geneva (UNIGE), Switzerland, led by Denis Jabaudon, a professor in the Department of Basic Neurosciences at the UNIGE Faculty of Medicine, have now deciphered this process. While neurons are anatomically different, their genetic programs remain very similar. It turns out that the variations arise during the molecular maturation of these neurons, which must follow a precise rhythm to form the correct connections.
Read the full story here
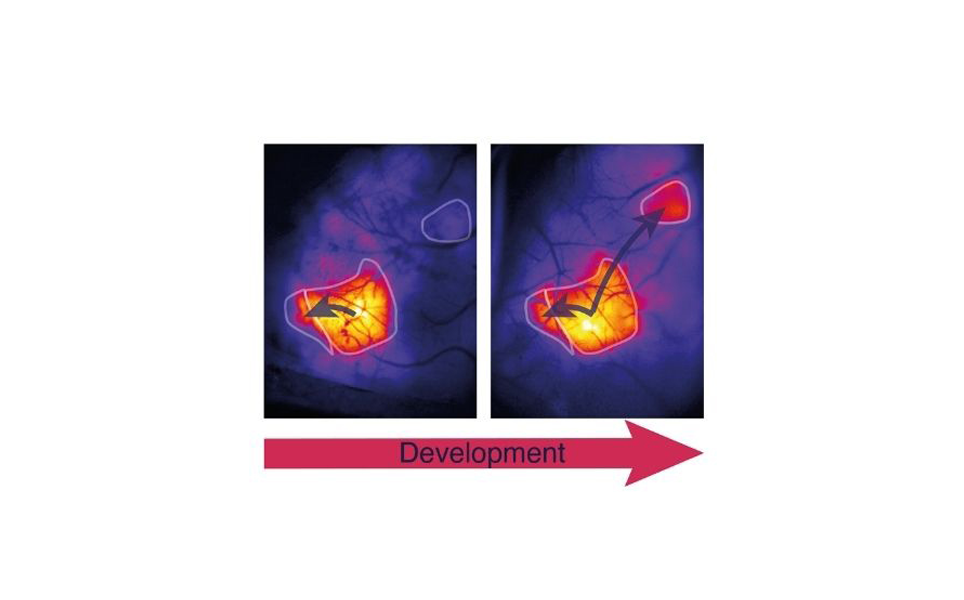
‘Dancing molecules’ successfully repair severe spinal cord injuries
A team of researchers at Northwestern University have established a new injectable therapy that harnesses “dancing molecules”, to reverse paralysis and repair tissue after severe spinal cord injuries.
In just four weeks, after researchers had administered a single injection to tissue surrounding the spinal cord of paralysed mice, the animals were able to walk once again. Once the therapy has been successful, within 12 weeks, the materials biodegrade into nutrients for the cells and eventually, without noticeable side effects, disappear from the body.
The key behind this new therapy is tuning the motion of molecules, so they are able to locate and appropriately engage constantly moving cellular receptors. Once this has been achieved and they are connected to the receptors, the moving molecules set off two cascading signals, both are essential to spinal cord repair. The first signal prompts the long tails of neurons in the spinal cord, called axons, to regenerate. The second signal aids neurons to survive after injury by triggering other cell types to proliferate, supporting the regrowth of lost blood vessels that feed neurons and critical cells for tissue repair.
Read the full story here
Georgia State researchers reveal surprising findings on how salt affects blood flow in the brain
In a first of its kind study, researchers at Georgia State University created a novel approach that unites surgical techniques and state-of-the-art neuroimaging, to investigate how blood flow to the hypothalamus changed in response to salt intake. Previous studies have detected a positive link between neuron activity and increased blood flow. However, the researchers found a decline in blood flow as the neurons became activated in the hypothalamus.
Dr. Javier Stern, professor of neuroscience at Georgia State said “The findings took us by surprise because we saw vasoconstriction, which is the opposite of what most people described in the cortex in response to a sensory stimulus,” The team called this surprising discovery “inverse neurovascular coupling,”
The research team intends to study this inverse neurovascular coupling mechanism in animal models, to establish whether it contributes to the pathology of salt-dependent hypertension.
Read the full story here
In the brain’s cerebellum, a new target for suppressing hunger
According to a new study led by J. Nicholas Betley, University of Pennsylvania, and Albert I. Chen, the Scintillion Institute, constant hunger associated with Prader Willi syndrome, a result in part to disordered signaling in the brain’s cerebellum - an area of the brain also accountable for motor control and learning.
The international research team used clues from Prader Willi patients, to direct investigations in mice that exposed a subset of cerebellar neurons that signals satiation after eating. According to Betley, when the researchers triggered these neurons, the scale of the effect “was enormous,”. The animals ate just as often as typical mice, but each of their meals was 50-75% smaller.
The study suggests that neurons in the cerebellum’s anterior deep cerebellar nuclei (aDCN) are involved in assisting animals to control their meal size.
Read the full story here

Balancing fear
To avoid harmful behaviours, such as panic attacks or exaggerated risk tasking, fear must be regulated. A new study from researchers at the Max Planck Institute of Neurobiology, provides insight on this.
In mice, the brain depends on the body’s feedback to control fear. The brain’s insular cortex strongly reacts to stimuli signaling danger. But, when the body freezes as a direct reaction to fear, the heartbeat slows down, leading to attenuated insular cortex activity. Processing these opposite signals aids the insular cortex to keep fear in balance. The body’s reactions are therefore actively used to regulate emotions and are far more than passive emotional responses.
Read the full story here
UCI-led study finds single molecule within a specific plant used by Native Americans can treat both pain and diarrhoea
In collaboration with the US National Parks Service, a University of California Irvine led study, found plants that activated the KCNQ2/3 potassium channel, a protein that passes electrical impulses in the brain and other tissues, showed a long history of use by Native Americans as topical analgesics.
KCNQ2/3 is present in nerve cells that recognise pain, and its activation would be projected to soothe pain by disfavouring transmission of the pain signal. The discovery came when the research team found that the same plant extracts that trigger KCNQ2/3, have the reverse effect on the related intestinal potassium channel, KCNQ1-KCNE3. Previous studies on modern medicines showed that KCNQ1-KCNE3 inhibitors can prevent diarrhea.
Geoffrey Abbott, PhD, a professor in the Department of Physiology and Biophysics at the UCI School of Medicine said, “this study illustrates how much there is still to learn from the medicinal practices of Native Americans, and how, by applying molecular mechanistic approaches we can highlight their ingenuity, provide molecular rationalizations for their specific uses of plants, and potentially uncover new medicines from plants.”
Read the full story here

Banner Credit: Georgia State University.

)