
Recent advances in optogenetics research
Here, we have collated a variety of recently published neuroscience research that used optogenetics. These include a less-invasive way to deliver light deeper into the mouse brain, using light as a potential therapy to treat chronic pain and using optogenetics to map cells in the brain.
Optogenetics is a research technology that enables neuroscientists to use light to control neurons with high spatial and temporal resolution. Specific neurons in the brain are genetically modified to express light-sensitive proteins. When light hits these neurons, the proteins are activated, causing the neuron to fire. Scientists are therefore able to specifically switch signalling cascades on and off using light.
Using optogenetics, neuroscientists can precisely measure the effects of turning a set of neurons on or off in real time in freely moving animals. They can see the behaviours they contribute to, allowing them to infer what they are needed for and identify cells that contribute to or remedy a disease state.
Read more about optogenetics here
Less-invasive ways of using optogenetics
Light delivery in optogenetics often uses invasive optical fibres, which can cause tissue damage and alter behaviour. Developing ways to less-invasively, and even non-invasively, deliver light to specific neurons in the brain, will improve reliability of research as well as animal welfare.
Deep-brain exploration with nanomaterial
A team of researchers have developed a way to less-invasively deliver light deeper into the mouse brain. The team of scientists from the RIKEN Brain Science Institute, the National University of Singapore, the University of Tokyo, John Hopkins University and Keio University used upconversion nanoparticles. These nanoparticles absorb near-infrared laser light and then emit visible photons of blue/green wavelengths, which activate or inhibit optogenetically modified neurons.
The near-infrared light is delivered from outside the skull, negating the need for optical fibres to be implanted into the brain, and can penetrate deeper into brain tissue. By emitting visible wavelengths of light, the upconversion nanoparticles remotely deliver light to cells that express light-sensitive proteins, enabling near-infrared light to be used to activate or inhibit cells deeper in the brain.
In studies, the nanoparticles appeared to be suitable for long-term use, due to their stability and biocompatibility. It is hoped that in the future, this technique could be used to treat movement disorders, such as Parkinson’s disease.
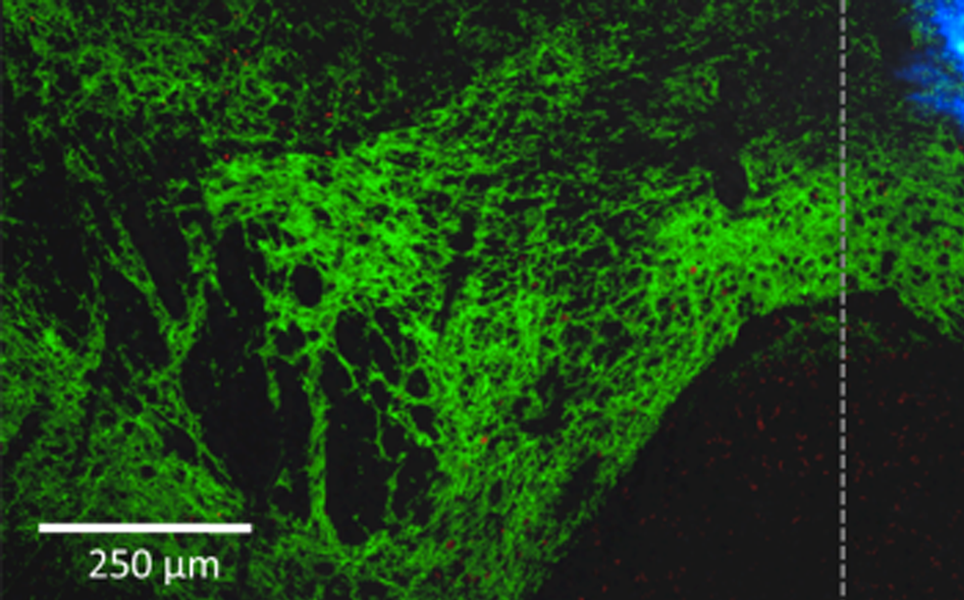
Deeper light delivery
Tailoring light delivery for optogenetics by modal demultiplexing in Tapered Optical Fibers
Tapered Optical Fibers enable light to be delivered to the living brain with reduced invasiveness and under tight spatiotemporal control. Unlike common light delivery methods, Tapered Optical Fibers can deliver light to regions deeper than 1mm without being highly invasive. Previously these researchers, in a collaborative framework between the Center for Biomolecular Nanotechnologies of the Italian Institute of Technology (Lecce, Italy) and Harvard Medical School (Boston), developed a thin Tapered Optical Fiber that could administer light to brain regions more than 1.8mm deep in the dorso-ventral direction.
Here, the researchers analysed the use of high-numerical aperture (NA) fibers to obtain wide volume or spatially-selective light delivery over 3mm, which can cover entire brain regions of several animal models. The results give quantitative data on Tapered Optical Fibers as light delivery tools which can be used to design future experiments using a range of opsins in multiple brain regions.
Read more
A Scientifica SliceScope was used to detect fluorescence emission and capture fluorescence images in this research.
Using optogenetics to treat chronic pain
As optogenetics can be used to switch neurons on and off, it has great promise as a potential method of treating chronic pain. By selectively switching off pain-sensing neurons, specific pain signals can be deactivated, eliminating sensations of pain.
Turning the light switch on to treat chronic pain
Researchers at Washington University School of Medicine in St Louis and the University of Illinois at Urbana-Champaign used optogenetics for the first time to reduce bladder pain in mice. These results offer hope for treating interstitial cystitis (also known as bladder pain syndrome) which affects millions.
The mice were bred to express light-sensitive proteins in their pain-sensing neurons and were implanted with wirelessly controlled LED devices that delivered light to the bladder. This enabled the researchers to activate the light-sensitive proteins using LED light; switching on the light activated the proteins, which silenced the neuronal signals, resulting in pain relief.
This method could be new way to treat pain in humans without using drugs, which can be addictive.
Switching off pain
Managing chronic pain with light
After discovering that the nerve cells in the skin that respond to gentle touch are the same cells that cause neuropathic pain, Scientists at EMBL Rome have developed a light-sensitive chemical that selectively binds to these nerve cells in the skin.
The chemical is injected into the affected area of skin and then illuminated by near-infrared light. This causes the nerve cells to withdraw from the skin’s surface, leading to pain relief. Other cell types, responsible for other sensations such as cold, vibration and normal pain, are not affected by the light treatment. The skin is only desensitised to gentle touch.
The pain relief lasts for a few weeks, after which the nerve endings grow back. This research was carried out in mice, but, as human skin contains the same cells, it is hoped the technique can one day be used to treat humans who suffer from neuropathic pain. The team are now actively looking for new collaborations to develop this method further.
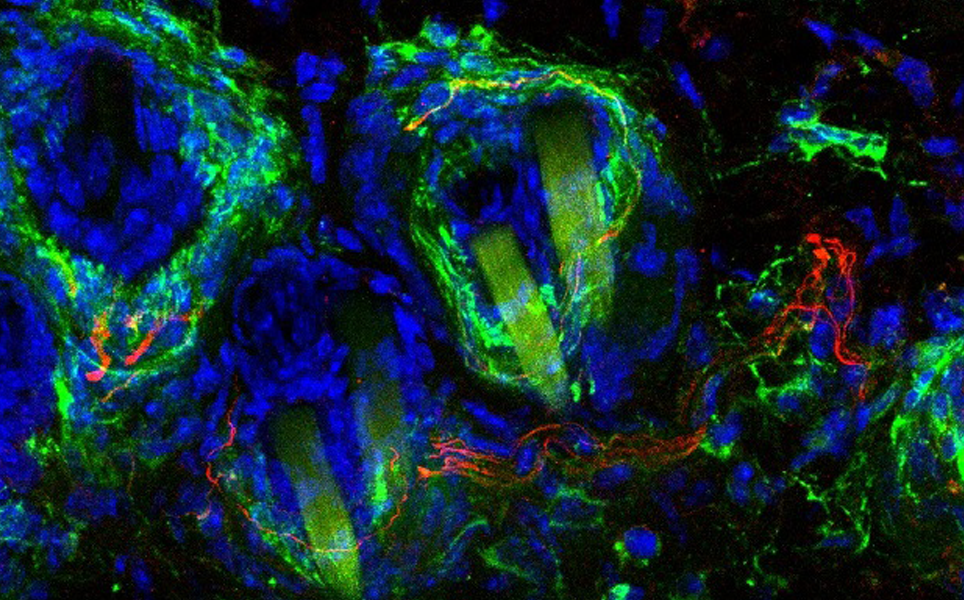
Chronic pain relief
Discoveries using optogenetics
The ability to activate and inactivate neurons using optogenetics has enabled researchers to see the role they have in normal functions, for example, learning and memory, vision and anxiety, as well as their implications in diseases.
Scientists identify connection between dopamine and behaviour related to pain and fear
Scientists at the University of Maryland School of Medicine used optogenetics to find a direct link between dopamine and avoidance-behaviour related to pain and fear. The researchers optogenetically stimulated dopamine neurons in the nucleus accumbens of rats to release either more or less dopamine. They found that animals with high levels of dopamine in this brain region learnt to avoid small electric shocks more quickly than those with lower levels of dopamine, indicating that dopamine drives animals to avoid unpleasant or painful situations and stimuli.
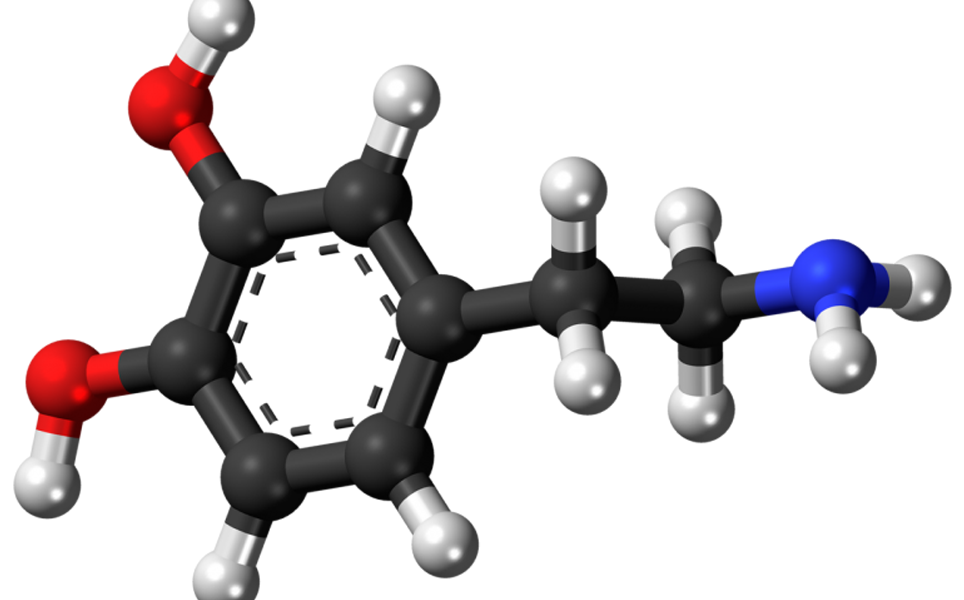
Learn more
A Vital Pause: Neurons in the Brain’s Striatum May Help Regulate Response to Unexpected Stimuli
In a study by Okinawa Institute of Science and Technology Graduate University, scientists used optogenetics to investigate the role of cholinergic interneurons in the striatum in regulating the response to unexpected stimuli.
Cholinergic interneurons in the striatum are in a near-constant state of activity, but if the brain receives an unexpected stimulus from the environment, these interneurons briefly stop firing. Optogenetics enabled the researchers to switch the cholinergic interneurons on and off as mice moved around their cage. They discovered that when they paused cholinergic interneuron firing, the spiny projection neurons that they are connected to were stimulated less. These spiny projection neurons send impulses from the striatum to the rest of the brain, causing it to respond to the stimuli, therefore pauses in cholinergic interneurons may mediate how animals respond to unexpected stimuli.
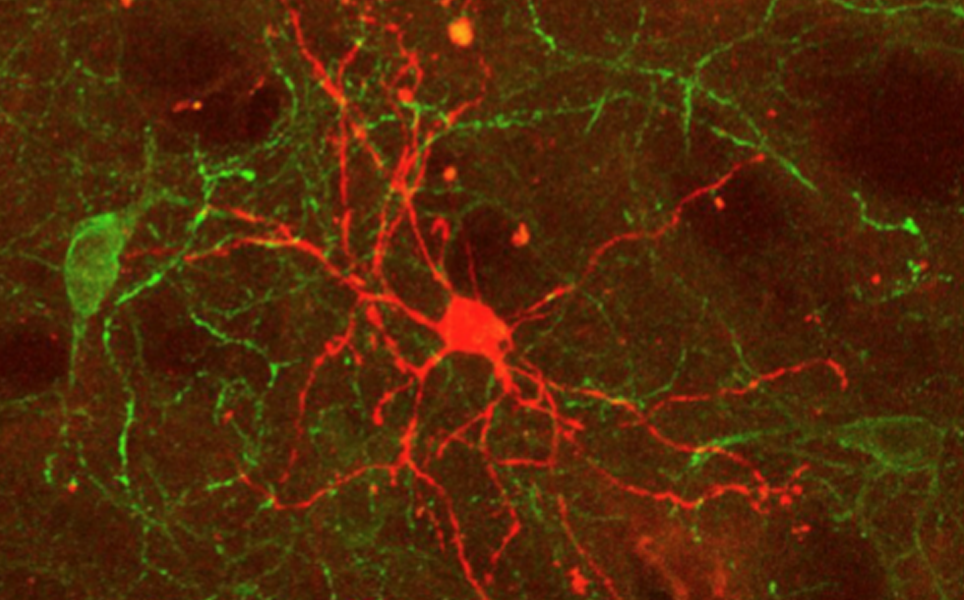
Expect the unexpected
Olfactory bulb acetylcholine release dishabituates odor responses and reinstates odour investigation
At the University of Tennessee Health Science Center, researchers used optogenetics to study the role of acetylcholine in dishabituation of glomeruli in the olfactory bulb to prolonged odours.
The team used optogenetics to control the release of acetylcholine in the olfactory bulb during prolonged exposure to odour. They discovered that acetylcholine can rapidly enhance the post-synaptic glomerular responses that are habituated to an odour and increase the salience of the odour. They also discovered that this response in the olfactory bulb can be blocked by a cholinergic antagonist.
Sense of smell
A Scientifica SliceScope was used for imaging in both awake and anaesthetised mice.
Neurons use a single switch to decide whether to make or break new connections
Optogenetics were utilised in this research to investigate how neuronal protrusions called filopodia 'know' where to make connections and how they make stable connections with neighbouring neurons.
The researchers, from Jefferson (Philadelphia University and Thomas Jefferson University), found that the same molecule can both repel and connect synapses. The receptor, EphB2 kinase, on the tip of the filopodium receives signals from outside the cell and relays these inside the cell. Using a light-activated version of EphB2 kinase, they found that when EphB2 kinase received a fast activation signal, the filopodium would retract, rejecting any possible connections. However, when it received a slow activation signal, it enabled filopodia to make stable connections.
Making connections
Specific set of nerve cells controls epileptic seizures' spread through brain
Researchers at Stanford University have found that a small set of nerve cells in the hippocampus, called mossy cells, prevent the spread of seizures that originate in the hippocampus. The hippocampus is the main focus site for seizures in temporal lobe epilepsy. Therefore, drugs that target mossy cells may one day be able to reduce the number of seizures patients suffer from, improving their quality of life.
The researchers engineered mice so their hippocampal mossy cells responded to pulses of light. Blue light caused them to fire, while amber light stopped them firing. Inhibiting mossy cells lead to an increase in the number of seizures that spread from the focus to other areas of the brain. Activating the mossy cells reduced the number of visible seizures but had no effect on seizures that remain in the focal point and don’t spread to other regions of the brain.
More here
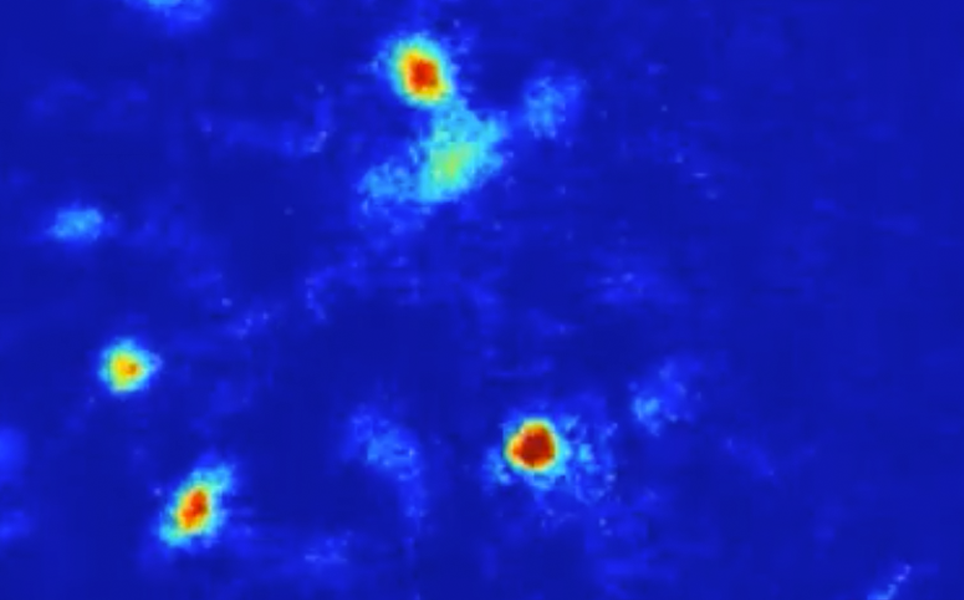
‘Anxiety cells’ identified in the brain’s hippocampus
Neuroscientists at Columbia University Irving Medical Center and UC San Francisco have identified cells in the hippocampus of mice that fire in frightening situations. The cells signal to other parts of the brain, initiating anxious behaviours. This discovery could lead to the development of treatments that target these cells to reduce anxiety.
The team inserted a miniature microscope into the brains of mice, enabling them to record the activity of hundreds of hippocampal cells as they freely moved around their environment. When the mice were exposed to anxiety-provoking environments, specific cells in the ventral part of the hippocampus were active. These cells signal to the hypothalamus, a region in the brain that is known to control behaviours associated with anxiety. The mice seemed more anxious when the activity of these anxiety cells was higher.
Optogenetics was then used to turn the cells on and off. The researchers found that when these anxiety cells were silenced, the mice stopped exhibiting fear-related behaviours; they would navigate onto elevated platforms and away from protective walls. Whereas, when the cells were activated, the mice exhibited more fear-related behaviours, even when they were in a safe environment.
Find out more
Therapeutic developments
Clinical trials are underway for using optogenetics to restore light sensitivity to the retinas of patients suffering from retinitis pigmentosa. It is also hoped that in the future optogenetics can be used to develop more effective cochlear implants, improve deep brain stimulation and, as described above, treat chronic pain.
Engineers create most efficient red light-activated optogenetic switch for mammalian cells yet
Due to its longer wavelength, red light passes through tissue more easily and harmlessly, so is a safer way to control genes, and could be used in humans in the future. UC San Diego researchers have developed the most efficient optogenetic switch activated by red and far-red light to-date. The light-activated genetic switch has been successful in turning genes on and off in mammals, and could be used in gene therapies.
A new switch
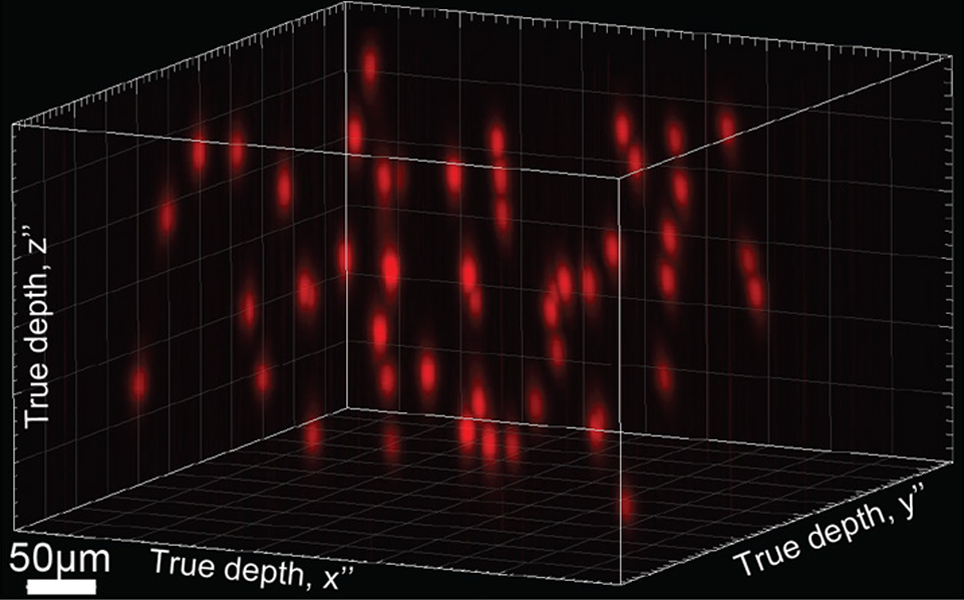
Editing brain activity with holography
Neuroscientists at UC Berkeley have developed a holographic brain modulator that can activate or suppress dozens or even thousands of cortical neurons at once using holographic projections. The team developed better optogenetic switches, that turn on strongly when activated and then quickly shut off, to be inserted into cells. These can be stimulated up to 50 times per second, similar to normal cortical firing rates. The team then developed the holographic projection system, called 3D-SHOT (Three-dimensional Scanless Holographic Optogenetics with Temporal focusing).
The researchers are hoping that by copying real patterns of brain activity, the projections will be able to mimic the brain activity when someone has seen or sensed something. By activating specific sets of neurons to stimulate an actual brain response, it is hoped that lost sensations caused by peripheral nerve damage can be replaced.
Mimicking the brain
High frequency neural spiking and auditory signalling by ultrafast red-shifted optogenetics
Channelrhodopsin mutants with fast closing kinetics have been developed by researchers at the Max Planck Institute, University Medical Center Göttingen and Friedrich Miescher Institute for Biomedical Research. The researchers focussed on Chrimson variants which are activated by red light, to reduce scattering and phototoxicity in tissues. Fast-closing channels are essential for many optogenetic experiments as many neurons in intact animals have high firing rates. For example, spiral ganglion neurons and cortical interneurons.
Optical cochlear implants utilise optogenetics to stimulate optogenetically-modified spiral ganglion neurons. The researchers demonstrated that the fast Chrimson mutants can be used to stimulate these neurons in optical cochlear implants to restore auditory nerve activity in deaf mice. They could therefore be used in the future for neuroscience research and medical applications, such as hearing restoration.
Listen out!
A Scientifica SliceScope was used in this research for in-vitro fluorescence imaging of brain slices.
Please send all holographic projections, mossy cells, upconversion nanoparticles, comments and suggestions to [email protected]
Banner image credit: EMBL Rome
Sign up to receive our latest news
Find out about Scientifica's latest product releases, company news, and developments through a range of news articles, customer interviews and product demonstration videos

)