Memory encoding: Synaptic plasticity requires specific coordinated activation
Scientists at the University of Bristol have shown that associative synaptic plasticity involves a complex intracellular signalling cascade requiring the activation of separate Ca2+ sources and metabotropic glutamate receptors.
Principal investigator, Dr Jack Mellor, from Bristol’s Centre for Synaptic Plasticity, said: “Our research shows that it is not simply the local concentration of calcium ions within single dendritic spines that determines whether a synapse becomes stronger or weaker. There is a much more complex cascade of feedback loops involved in the regulation of synaptic plasticity. This is important because it enables neuromodulator systems to control the triggers for synaptic plasticity.”
According to the paper, published in Nature Communications, long-term potentiation (LTP) at glutamatergic synapses in dendritic spines depends upon the activation of postsynaptic NMDA receptors (NMDARs) followed by voltage-sensitive Ca2+ channels (VSCCs). Furthermore, inhibition of SK channels by group 1 metabotropic glutamate receptors (mGluR1) activation is also required for lasting synaptic changes.
On the postsynaptic membrane of glutamatergic synapses on dendritic spines, an initial depolarisation of the postsynaptic spine is initiated by activation of ionotropic glutamate receptors. This depolarisation can be further amplified by the opening of voltage-gated calcium channels. NMDARs also only open when the neuron is already depolarised, increasing Ca2+ influx.
This creates a positive feedback loop that will eventually lead to cell toxicity and death if not regulated. Regulation comes in the form of SK channels, which pump K+ ions out of the cell when intracellular Ca2+ gets too high. However, the opening of SK channels inhibits synaptic plasticity, which depends on sustained depolarisation.
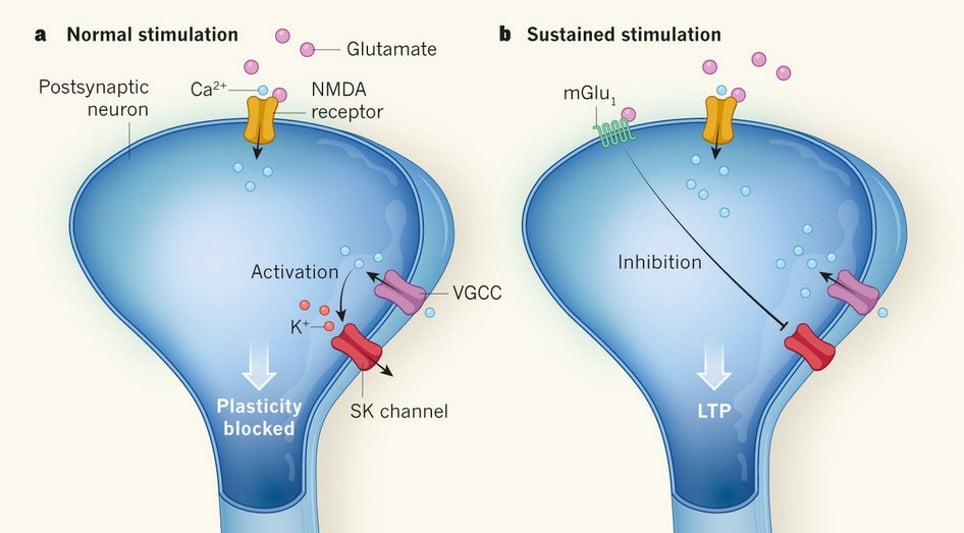
Synaptic plasticity mechanism in dendritic spines (Credit: Nature/Thomas G. Oertner)
The Nature Communications paper demonstrated that repeated activation of pre- and post-synaptic neurons induced LTP, but required the activity of mGluR1. These receptors act by inhibiting SK channels, leading to a sustained depolarisation and enhanced Ca2+ influx.
An important, and still unanswered question is how these mechanisms converge to determine the strength and direction of synaptic plasticity.
Dr Mellor said: “Scientifica’s Multiphoton Imaging System provided us with an integrated whole-cell patch clamp and calcium imaging system that enabled us to measure both the calcium response within single dendritic spines and the outcome of those calcium responses in terms of synaptic plasticity."
Whole-cell patch-clamp recordings were made from CA1 pyramidal neurons visualised under IR-DIC on a SliceScope Pro 6000/Multiphoton Imaging System. This same system was used for two-photon calcium imaging using dual channel fluorescence.
Paper References:
Tigaret C. M., Olive V., Sadowski J. H. L. P., Ashby M. C., Mellor J. R. Coordinated activation of distinct Ca2+ sources and metabotropic glutamate receptors encodes Hebbian synaptic plasticity Nature Communications (2016) doi: 10.1038/ncomms10289
Gee C.E., Oertner T.G. Neurobiology: Pull out the stops for plasticity Nature (2016) doi: 10.1038/529164a
Banner image credit: University of Bristol/Cezar Tigaret

)