
Making connections: The role of adhesion molecules in synaptic function
By Rachel Jackson
Dr Joris de Wit from the VIB-KU Leuven Center for Brain & Disease Research recently gave a talk as part of the NEUReka! seminar series at the Centre for Developmental Neurobiology, King’s College London. He explained the importance of adhesion molecules in defining the structure and function of synaptic connections.
Within the brain, information is transferred between neurons at specialised synaptic connections. To create a working neural circuit, synapses must be made between the appropriate neurons and have specific functional properties. Given that there are billions of neurons in the brain, each making thousands of synaptic connections, this is a complex task. Synaptic adhesion molecules are important in this process, mediating cell-cell recognition and physically connecting pre- and postsynaptic partners by binding across the synaptic cleft. While several families of adhesion molecules have been identified, there is still much to discover about these proteins, including the relationships between different adhesion molecules and their influence on synaptic function, topics which Dr de Wit discussed.
Glypican-4 is a presynaptic adhesion molecule that had previously been identified and found to bind to the postsynaptic molecule LRRTM4, expressed on dendrites of the granule cells of the hippocampus (de Wit et al, 2013). Glypican-4 is also expressed by the granule cells themselves at the presynaptic surface of their output synapses, the mossy fibre boutons. However, mossy fibres form synapses with cells in the CA3 area of the hippocampus and the target binding partner in this region had not been identified. By capturing all of the proteins that bind to glypican-4, Dr de Wit’s lab discovered a new postsynaptic partner, GPR158, which they found was expressed in the CA3 target area. In CA3 neurons which lack GPR158, synapse density was increased in the proximal dendrite but not the distal regions, suggesting that GPR158 may specify not only the target cells but also the subcellular location that mossy fibre boutons contact. The GPR158 knockout synapses were smaller than wild-type synapses and showed decreased responses when the mossy fibre was stimulated, indicating that GPR158 is important not only in specifying this connection but also in its correct function. Furthermore, the loss of GPR158 in CA1 cells did not replicate the phenotype caused by loss of LRRTM4 in granule cells (Siddiqui et al, 2013), showing that despite the common presynaptic partner, these adhesion molecule pairs act differentially on synaptic function.
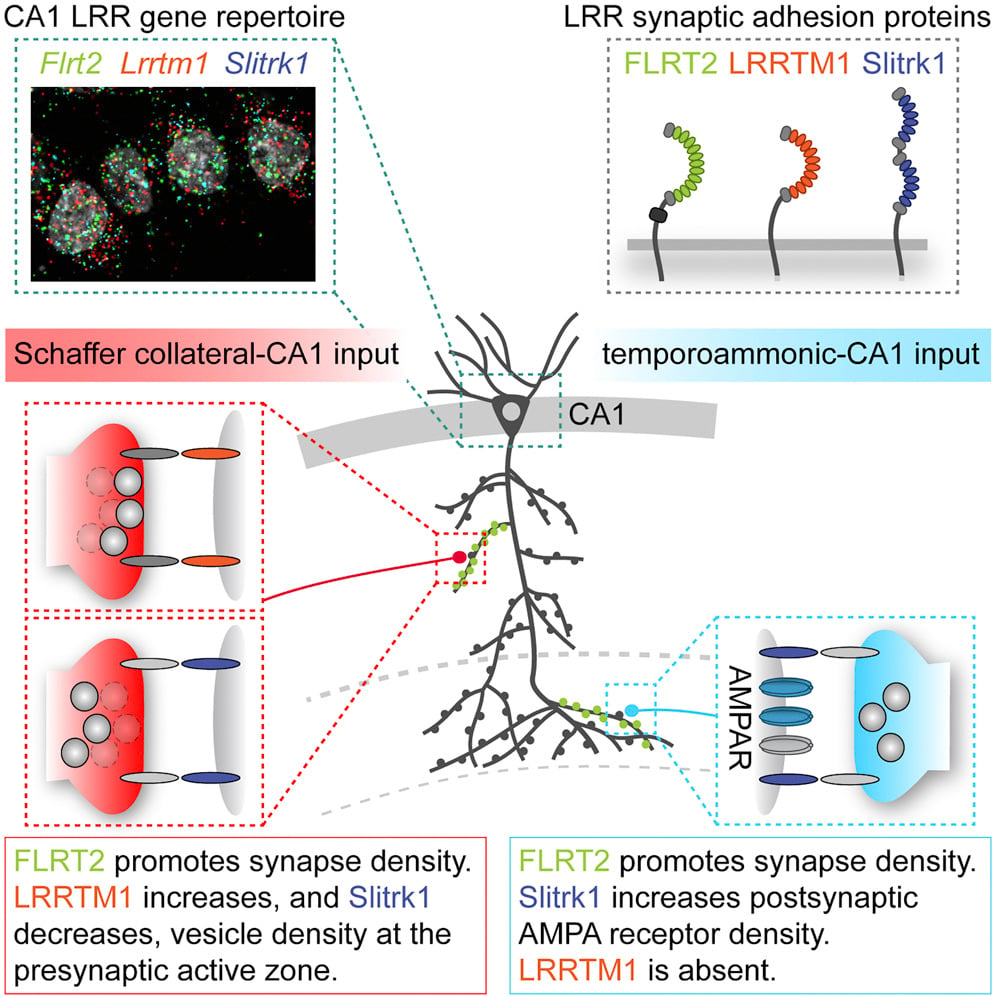
In another study, Dr de Wit and his team investigated why there may be several adhesion molecules expressed in the same neuron, focussing on those of the LRR protein family, for which there is a high diversity in mammalian cells (Schroeder et al, 2018 and see Figure 1). In the CA1 area of the hippocampus, 95% of pyramidal neurons express the postsynaptic proteins FLRT, LRRTM1 and Slitrk1, which each belong to a different LRR subfamily. However, the pattern of expression is different between proximal and distal regions of the dendrite, which receive inputs from different areas of the brain. All three proteins are expressed at proximal Schaffer collateral inputs that come from area CA3, while only FLRT2 and Slitrk1 are expressed at the distal temporoammonic (TA) inputs that originate in the entorhinal cortex. The researchers then used viruses to express shRNAs that knocked down each protein individually and used EM reconstruction and electrophysiology to examine the changes in synaptic structure and function that occurred in infected neurons. They found that FLRT2 played the same role in promoting synapse density in both proximal and distal regions, but surprisingly that Slitrk1 function was different between the two regions. At the Schaffer collateral inputs, knockdown of Slitrk1 caused an increase in the number of vesicles docked at the active zone of the presynaptic terminal, whilst at the TA inputs the knockdown of Slitrk1 caused a decrease in postsynaptic AMPA receptors. In line with its expression pattern, knockdown of LRRTM1 only caused changes at the proximal Schaffer collateral inputs where interestingly it was found to have the opposite effect to knockdown of Slitrk1. When all of these proteins were knocked down in the same neuron the cumulative phenotype was produced. Thus, these LRR proteins do not act redundantly but instead act in combination, influencing different aspects of synaptic structure and function in an input-specific manner.
There are many layers of complexity in synaptic connectivity; investigating how the intricate patterns of synapses are defined reveals rich repertoires of adhesion molecules, which themselves act in complex context-dependent combinations. Studying these molecular mechanisms further will aid our understanding of synaptic function and give greater insights into neurodevelopmental and neurodegenerative diseases, in which synaptic dysfunction is a key feature.
References
de Wit J, O’Sullivan ML, Savas JN, Condomitti G, Caccese MC, Vennekens KM, Yates JR 3rd, Ghosh A (2013) Unbiased discovery of glypican as a receptor for LRRTM4 in regulating excitatory synapse development. Neuron 79(4):696-711
Schroeder A, Vanderlinden J, Vints K, Ribeiro LF, Vennekens KM, Gounko NV, Wierda KD, de Wit J (2018) A Modular Organization of LRR Protein-Mediated Synaptic Adhesion Defines Synaptic Identity. Neuron 99(2):329-344.e7.
Siddiqui TJ, Tari PK, Connor SA, Zhang P, Dobie FA, She K, Kawabe H, Wang YT, Brose N, Craig AM (2013) An LRRTM4-HSPG complex mediates excitatory synapse development on dentate gyrus granule cells. Neuron 79(4):680-95
Scientifica supports the NEUReka! Seminar Series to help them book speakers from top research institutions around the world.
Are you interested in writing a Neurowire blog post?
Please email [email protected] for more information. Read examples of other guest blog posts here.
Find out about Scientifica's latest product releases, company news, and developments through a range of news articles, customer interviews and product demonstration videos.

)