
At the bench: Auditory coding at the inner hair cell synapse
By Dr Régis Nouvian
Inner hair cells (IHCs), the auditory sensory cells of the cochlea – the organ of hearing – transduce sound stimulation into a neural message. At their apical side, the IHCs harbour a staircase of actin-rich microvilli called stereocilia, where the mechanotransducer (MET) channels are located (Peng et al., 2011). The precise nature of the MET channel is still unknown, but the search for its molecular correlate is an intense field of investigation (Fettiplace and Kim, 2014). Incoming sound stimulation deflects the stereocilia bundle, opening the MET channels, which depolarise the hair cell. At the basolateral side, the hair cell depolarization opens the calcium channels leading to the influx of calcium required for synaptic vesicle fusion and glutamate release (Glowatzki et al., 2008). Activation of afferent auditory nerve fibres then conveys the acoustic information to the cochlear nuclei.
IHCs display very peculiar features. Before the onset of hearing, they fire spontaneous calcium action potential (Kros et al., 1998; Johnson et al., 2012) (Nouvian et al., 2015), thought to refine the ascending auditory pathway (Tritsch et al., 2010; Clause et al., 2014). At the onset of hearing, the action potential firing stops as the ionic conductances of the IHCs change, allowing the incoming sound stimulation to generate a receptor potential, which in turn governs the neurotransmitter release (Palmer and Russell, 1986; Kros et al., 1998). In our lab, patch-clamp recordings enable us to understand the action potential firing of developing IHCs and the calcium-triggered exocytosis.
Action potential firing in the cochlea
Before the onset of hearing (P10-P12 in the mouse), the cochlea is not capable of transducing incoming sound stimulation, as the ear canal is closed and the cochlear structures are still in an immature state. The spiking activity of the developing hair cells, therefore, provides a useful means to elicit the activation of the afferent auditory nerve fibres (Tritsch et al., 2007; Wang et al., 2015). As each single action potential is associated with the release of glutamate onto the afferent auditory nerve fibres (Beutner and Moser, 2001; Marcotti et al., 2003). Such activity is thought to consolidate and refine the synapses along the ascending auditory pathways (Tritsch et al., 2010; Clause et al., 2014). The discharge rate is also under the control of cholinergic efferent fibres, which originate from the brainstem and project onto the immature IHCs. Acetylcholine release activates the α9α10 nicotinic receptor, which in turn opens the SK2 potassium channel to hyperpolarize the IHCs (Glowatzki and Fuchs, 2000; Katz et al., 2004; Goutman et al., 2005; Zorrilla de San Martín et al., 2010; Johnson et al., 2011; Roux et al., 2011; Sendin et al., 2014). Using perforated patch-clamp recordings, we track the pattern of the spiking activity in the developing IHCs over an extended period. Thus, we demonstrated that the IHCs fire action potential in a burst manner, which becomes highly stereotyped at the end of the first postnatal week (Sendin et al., 2014). In the future, we aim at investigating in more details the mechanism underlying IHC activity and regulation using pharmacological tools and mutant mice.
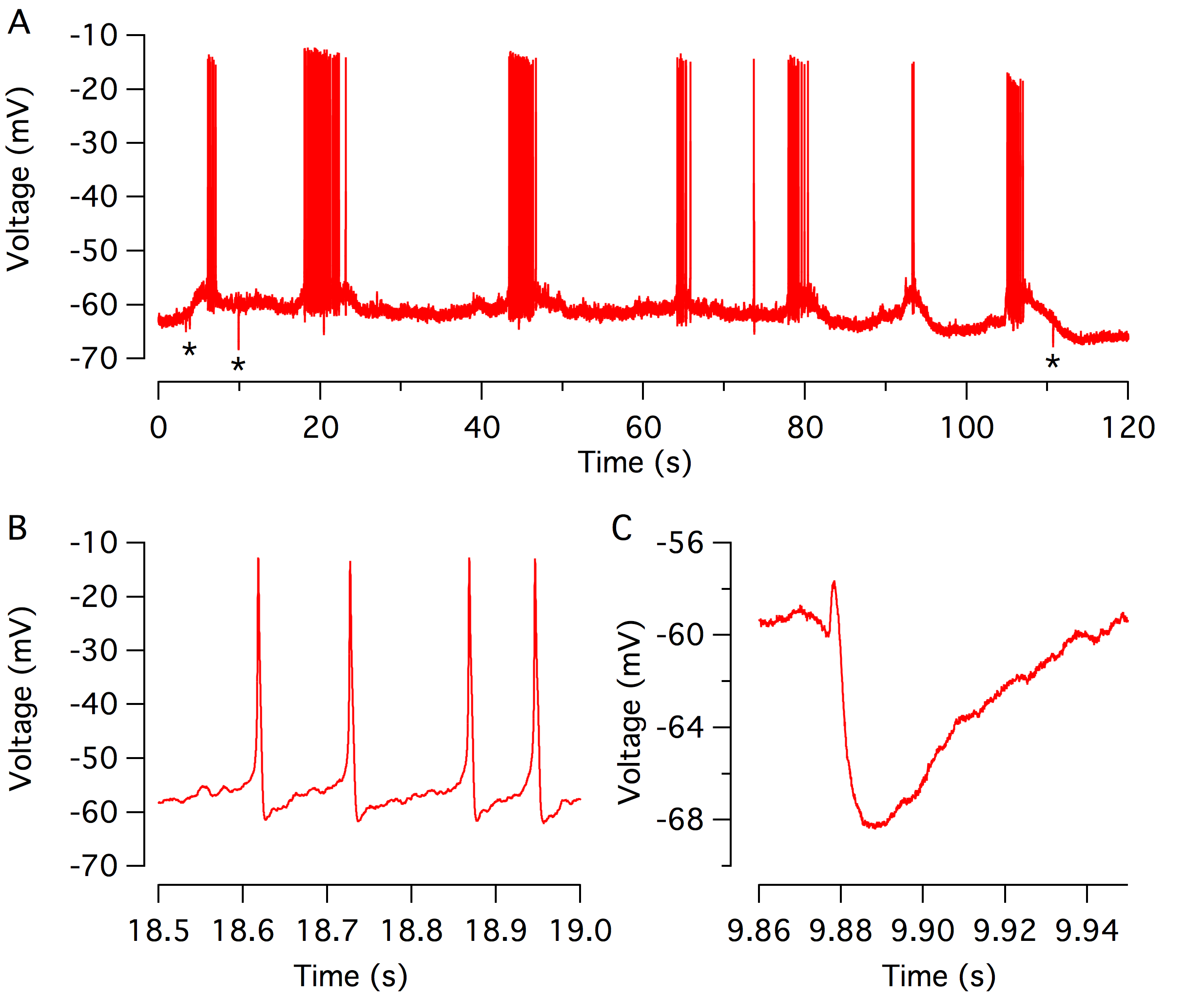
Action potential firing in developing inner hair cells. (A) Spontaneous spiking activity from a single IHC recorded using perforated patch-clamp in current-clamp mode (Iinj = 0 pA) near physiological temperature (37°C). Spiking activity of the developing IHC consists of bursts of action potential flanked by silent segments. Stars indicate inhibitory postsynaptic potentials driven by acetylcholine release from olivocochlear efferents. (B-C) expanded scale of the train of an action potential (B) and inhibitor post-synaptic potential (C).
Calcium-triggered exocytosis
After the onset of hearing, the capabilities of hair cells to encode the acoustic information are astonishing. The hair cells can sustain a phase-locked response of up to 2 kHz over an extended period. This peculiar feature requires extreme specialisation in the molecular determinants of the stimulation-secretion. Therefore, the synapses of the IHCs contain a large organelle, called the synaptic ribbon, which concentrates the glutamate-filled synaptic vesicles (Nouvian et al., 2006). Although some essential proteins, such as Ribeye - the major component of the ribbon - (Schmitz et al., 2000; Maxeiner et al., 2016), Bassoon - that anchors the ribbon to the plasma membrane - (Khimich et al., 2005; Frank et al., 2010), Vglut3 – the intravesicular glutamate transporter (Ruel et al., 2008; Seal et al., 2008) - or Otoferlin - that promote the synaptic vesicle replenishment - (Roux et al., 2006; Pangršič et al., 2010) have been identified, the full picture of the synaptic machinery has not yet been determined. Indeed, the exocytosis in hair cells seems to be independent of neuronal SNARES proteins (Nouvian et al., 2011), Complexins (Strenzke et al., 2009) and Munc13 and CAPS priming factors (Vogl et al., 2015).
We identify the molecular make-up of the IHCs ribbon synapse using pharmacological or molecular biology methods combined with membrane capacitance measurements, as a proxy of synaptic vesicle exo-endocytosis. Using this approach, we recently showed that the disruption of the actin filaments by Latrunculin A or Cytochalasin D increases the calcium-triggered synaptic vesicles exocytosis (Guillet et al., 2016). In our experiments, drugs were infused into the hair cell through a patch pipette. Thus, patch-clamp recordings provide us with clear-cut information to highlight the role of the protein of interest in the hair cell transmitter release. Another way to determine the synaptic machinery consists of using transgenic models, in which the protein of interest has been knocked out.
Additionally, the contribution of the proteins in sound coding is tested at the system level using electrocochleography, which helps us to grasp the hair cells potential receptor in vivo, and using the auditory brainstem response, which reflects the synchronous activation of the ascending auditory pathway. Because the alteration in transmitter release leads to auditory deficits, knowing the role of the different hair cell synaptic proteins is an essential requisite for future hearing restoration (Moser and Starr, 2016).
About the author:
Dr Régis Nouvian received his PhD in Neurosciences in 2004 under the supervision of Jean-Luc Puel (Montpellier, France). He then joined the lab of Tobias Moser as a post-doctoral fellow (Göttingen, Germany). In 2008, Dr Nouvian was hired by the CNRS as a researcher to work at the Institute for Neurosciences of Montpellier (Inserm U1051).
Find out more on Dr Nouvian's website
Banner Image Credit: Dr Sonja Pyott, The University Medical Center Groningen (UMCG)
Paper references:
Beutner D., Moser T. (2001) The presynaptic function of mouse cochlear inner hair cells during development of hearing The Journal of Neuroscience 21:4593–4599
Clause A., Kim G., Sonntag M., Weisz C.J.C., Vetter D.E., Rübsamen R., Kandler K. (2014) The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement Neuron 82:822–835
Fettiplace R., Kim K.X. (2014) The physiology of mechanoelectrical transduction channels in hearing Physiological Review 94:951–986
Frank T., Rutherford M.A., Strenzke N., Neef A., Pangršič T., Khimich D., Fejtova A., Gundelfinger E.D., Liberman M.C., Harke B., Bryan K.E., Lee A., Egner A., Riedel D., Moser T. (2010) Bassoon and the synaptic ribbon organize Ca²+ channels and vesicles to add release sites and promote refilling Neuron 68:724–738
Glowatzki E., Fuchs P.A. (2000) Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea Science 288:2366–2368
Glowatzki E., Grant L., Fuchs P. (2008) Hair cell afferent synapses Current Opinions in Neurobiology 18:389–395
Goutman J.D., Fuchs P.A., Glowatzki E. (2005) Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat The Journal of Physiology 566:49–59
Guillet M., Sendin G., Bourien J., Puel J-L., Nouvian R. Actin Filaments Regulate Exocytosis at the Hair Cell Ribbon Synapse The Journal of Neuroscience (2016) 36:649–654
Johnson S.L., Eckrich T., Kuhn S., Zampini V., Franz C., Ranatunga K.M., Roberts T.P., Masetto S., Knipper M., Kros C.J., Marcotti W. (2011) Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells Nature Neuroscience 14:711–717
Johnson S.L., Kennedy H.J., Holley M.C., Fettiplace R., Marcotti W. (2012) The resting transducer current drives spontaneous activity in prehearing Mammalian cochlear inner hair cells The Journal of Neuroscience 32:10479–10483
Katz E., Elgoyhen A.B., Gómez-Casati M.E., Knipper M., Vetter D.E., Fuchs P.A., Glowatzki E. (2004) Developmental regulation of nicotinic synapses on cochlear inner hair cells The Journal of Neuroscience 24:7814–7820
Khimich D., Nouvian R., Pujol R., Tom Dieck S., Egner A., Gundelfinger E.D., Moser T. (2005) Hair cell synaptic ribbons are essential for synchronous auditory signalling Nature 434:889–894
Kros C.J., Ruppersberg J.P., Rüsch A. (1998) Expression of a potassium current in inner hair cells during development of hearing in mice Nature 394:281–284.
Marcotti W., Johnson S.L., Rusch A., Kros C.J. (2003) Sodium and calcium currents shape action potentials in immature mouse inner hair cells The Journal of Physiology 552:743–761
Maxeiner S., Luo F., Tan A., Schmitz F., Südhof T.C. (2016) How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release The EMBO Journal
Moser T., Starr A. (2016) Auditory neuropathy - neural and synaptic mechanisms Nature Reviews Neurology 12(3):135-49
Nouvian R., Beutner D., Parsons T.D., Moser T. (2006) Structure and function of the hair cell ribbon synapse The Journal of Membrane Biology 209:153–165
Nouvian R., Eybalin M., Puel J-L. (2015) Cochlear efferents in developing adult and pathological conditions Cell and Tissue Research 1(1):301-9
Nouvian R., Neef J., Bulankina A.V., Reisinger E., Pangršič T., Frank T., Sikorra S., Brose N., Binz T., Moser T. (2011) Exocytosis at the hair cell ribbon synapse apparently operates without neuronal SNARE proteins Nature Neuroscience 14:411–413
Palmer A.R., Russell I.J. (1986) Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells Hearing Research 24:1–15
Pangršič T., Lasarow L., Reuter K., Takago H., Schwander M., Riedel D., Frank T., Tarantino L.M., Bailey J.S., Strenzke N., Brose N., Müller U., Reisinger E., Moser T. (2010) Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells Nature Neuroscience 13:869–876
Peng A.W., Salles F.T., Pan B., Ricci A.J. (2011) Integrating the biophysical and molecular mechanisms of auditory hair cell mechanotransduction Nature Communications 2:523
Roux I., Safieddine S., Nouvian R., Grati M., Simmler M-C., Bahloul A., Perfettini I., Le Gall M., Rostaing P., Hamard G., Triller A., Avan P., Moser T., Petit C. (2006) Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse Cell 127:277–289
Roux I., Wersinger E., McIntosh J.M., Fuchs P.A., Glowatzki E. (2011) Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea The Journal of Neuroscience 31:15092–15101
Ruel J., Emery S., Nouvian R., Bersot T., Amilhon B., van Rybroek J.M., Rebillard G., Lenoir M., Eybalin M., Delprat B., Sivakumaran T.A., Giros B., Mestikawy S., Moser T., Smith R.J.H., Lesperance M.M., Puel J-L. (2008) Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice The American Journal of Human Genetics 83:278–292
Schmitz F., Königstorfer A., Südhof T.C. (2000) RIBEYE, a component of synaptic ribbons: a protein's journey through evolution provides insight into synaptic ribbon function Neuron 28:857–872
Seal R.P., Akil O., Yi E., Weber C.M., Grant L., Yoo J., Clause A., Kandler K., Noebels J.L., Glowatzki E., Lustig L.R., Edwards R.H. (2008) Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 57:263–275
Sendin G., Bourien J., Rassendren F., Puel J-L., Nouvian R. (2014) Spatiotemporal pattern of action potential firing in developing inner hair cells of the mouse cochlea Proceedings of the National Academy of Sciences 111:1999–2004
Strenzke N., Chanda S., Kopp-Scheinpflug C., Khimich D., Reim K., Bulankina A.V., Neef A., Wolf F., Brose N., Xu-Friedman M.A., Moser T. (2009) Complexin-I is required for high-fidelity transmission at the endbulb of Held auditory synapse The Journal of Neuroscience 29:7991–8004
Tritsch N.X., Rodríguez-Contreras A., Crins T.T.H., Wang H.C., Borst J.G.G., Bergles D.E. (2010) Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset Nature Neuroscience 13:1050–1052
Tritsch N.X., Yi E., Gale J.E., Glowatzki E., Bergles D.E. (2007) The origin of spontaneous activity in the developing auditory system Nature 450:50–55
Vogl C., Cooper B.H., Neef J., Wojcik S.M., Reim K., Reisinger E., Brose N., Rhee J-S., Moser T., Wichmann C (2015) Unconventional molecular regulation of synaptic vesicle replenishment in cochlear inner hair cells Journal of Cell Science 128:638–644
Wang H.C., Lin C-C., Cheung R., Zhang-Hooks Y., Agarwal A., Ellis-Davies G., Rock J., Bergles D.E. (2015) Spontaneous Activity of Cochlear Hair Cells Triggered by Fluid Secretion Mechanism in Adjacent Support Cells. Cell 163(6):1348-1359
Zorrilla de San Martín J., Pyott S., Ballestero J., Katz E. (2010) Ca(2+) and Ca(2+)-activated K(+) channels that support and modulate transmitter release at the olivocochlear efferent-inner hair cell synapse The Journal of Neuroscience 30:12157–12167
Banner Image Credit: Dr Sonja Pyott, The University Medical Center Groningen (UMCG)

)